The D-ring, not the A-ring, rotates in Synechococcus OS-B' phytochrome
- PMID: 24327657
- PMCID: PMC3908390
- DOI: 10.1074/jbc.M113.520031
The D-ring, not the A-ring, rotates in Synechococcus OS-B' phytochrome
Abstract
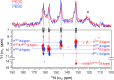
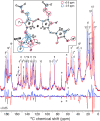
Phytochrome photoreceptors in plants and microorganisms switch photochromically between two states, controlling numerous important biological processes. Although this phototransformation is generally considered to involve rotation of ring D of the tetrapyrrole chromophore, Ulijasz et al. (Ulijasz, A. T., Cornilescu, G., Cornilescu, C. C., Zhang, J., Rivera, M., Markley, J. L., and Vierstra, R. D. (2010) Nature 463, 250-254) proposed that the A-ring rotates instead. Here, we apply magic angle spinning NMR to the two parent states following studies of the 23-kDa GAF (cGMP phosphodiesterase/adenylyl cyclase/FhlA) domain fragment of phytochrome from Synechococcus OS-B'. Major changes occur at the A-ring covalent linkage to the protein as well as at the protein residue contact of ring D. Conserved contacts associated with the A-ring nitrogen rule out an A-ring photoflip, whereas loss of contact of the D-ring nitrogen to the protein implies movement of ring D. Although none of the methine bridges showed a chemical shift change comparable with those characteristic of the D-ring photoflip in canonical phytochromes, denaturation experiments showed conclusively that the same occurs in Synechococcus OS-B' phytochrome upon photoconversion. The results are consistent with the D-ring being strongly tilted in both states and the C15=C16 double bond undergoing a Z/E isomerization upon light absorption. More subtle changes are associated with the A-ring linkage to the protein. Our findings thus disprove A-ring rotation and are discussed in relation to the position of the D-ring, photoisomerization, and photochromicity in the phytochrome family.
Keywords: Biophysics; Cyanobacteria; Molecular Biology; NMR; Photoisomerization; Photoreceptors; Phytochrome; Protein Structure; Structural Biology.
Figures

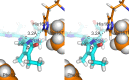
References
-
- Hughes J. (2010) Phytochrome three-dimensional structures and functions. Biochem. Soc. Trans. 38, 710–716 - PubMed
-
- Song C., Rohmer T., Tiersch M., Zaanen J., Hughes J., Matysik J. (2013) Solid-state NMR spectroscopy to probe photoactivation in canonical phytochromes. Photochem. Photobiol. 89, 259–273 - PubMed
-
- Wagner J. R., Brunzelle J. S., Forest K. T., Vierstra R. D. (2005) A light-sensing knot revealed by the structure of the chromophore-binding domain of phytochrome. Nature 438, 325–331 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

