Lanthanide probes for bioresponsive imaging
- PMID: 24328202
- PMCID: PMC3999228
- DOI: 10.1021/cr400477t
Lanthanide probes for bioresponsive imaging
Figures
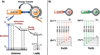

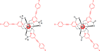

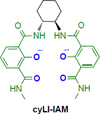


















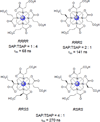



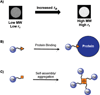



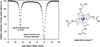










References
-
- Bünzli J-CG, Eliseeva SV. In: Springer Series on Fluorescence. Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects. Hänninen P, Härmä H, editors. Vol. 7. Springer Verlag: Verlag Berlin Heidelberg; 2011. pp. 1–46.
-
- Ramogida CF, Orvig C. Chem. Commun. 2013;49:4720. - PubMed
-
- Laurent S, Vander Elst L, Muller RN. Q. J. Nucl. Med. Mol. Imaging. 2009;53:586. - PubMed
-
- Hanaoka K. Chem. Pharm. Bull. 2010;58:1283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

