Redox-neutral α-arylation of amines
- PMID: 24328366
- PMCID: PMC3946350
- DOI: 10.1021/ol403431u
Redox-neutral α-arylation of amines
Abstract
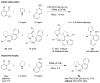
The direct α-arylation/N-alkylation of cyclic amines was achieved in a redox-neutral fashion under mild conditions. Transformations occur in the absence of any additives or are promoted by simple carboxylic acids.
Figures


References
-
-
Reviews on redox-economy and redox-neutral amine α-functionalization: Burns NZ, Baran PS, Hoffmann RW. Angew Chem Int Ed. 2009;48:2854.Newhouse T, Baran PS, Hoffmann RW. Chem Soc Rev. 2009;38:3010.Peng B, Maulide N. Chem Eur J. 2013;19:13274.
-
-
-
Selected reviews on amine α-functionalization: Murahashi SI. Angew Chem, Int Ed Engl. 1995;34:2443.Matyus P, Elias O, Tapolcsanyi P, Polonka-Balint A, Halasz-Dajka B. Synthesis. 2006:2625.Campos KR. Chem Soc Rev. 2007;36:1069.Murahashi SI, Zhang D. Chem Soc Rev. 2008;37:1490.Li CJ. Acc Chem Res. 2009;42:335.Yoo WJ, Li CJ. Top Curr Chem. 2010;292:281.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem Eur J. 2010;16:2654.Liu C, Zhang H, Shi W, Lei AW. Chem Rev. 2011;111:1780.Yeung CS, Dong VM. Chem Rev. 2011;111:1215.Sun CL, Li BJ, Shi ZJ. Chem Rev. 2011;111:1293.Wendlandt AE, Suess AM, Stahl SS. Angew Chem Int Ed. 2011;50:11062.Jones KM, Klussmann M. Synlett. 2012;23:159.Zhang C, Tang CH, Jiao N. Chem Soc Rev. 2012;41:3464.Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW. Chem Eur J. 2012;18:10092.Pan SC. Beilstein J Org Chem. 2012;8:1374.Platonova AY, Glukhareva TV, Zimovets OA, Morzherin YY. Chem Heterocycl Compd. 2013;49:357.
-
-
-
Selected recent examples of amine α-functionalization via Hshifts: Barluenga J, Fananas-Mastral M, Aznar F, Valdes C. Angew Chem Int Ed. 2008;47:6594.Zhang C, Murarka S, Seidel D. J Org Chem. 2009;74:419.Murarka S, Zhang C, Konieczynska MD, Seidel D. Org Lett. 2009;11:129.Mori K, Ohshima Y, Ehara K, Akiyama T. Chem Lett. 2009;38:524.Ruble JC, Hurd AR, Johnson TA, Sherry DA, Barbachyn MR, Toogood PL, Bundy GL, Graber DR, Kamilar GM. J Am Chem Soc. 2009;131:3991.Cui L, Peng Y, Zhang L. J Am Chem Soc. 2009;131:8394.Murarka S, Deb I, Zhang C, Seidel D. J Am Chem Soc. 2009;131:13226.Dunkel P, Turos G, Benyei A, Ludanyi K, Matyus P. Tetrahedron. 2010;66:2331.Zhou G, Zhang J. Chem Commun. 2010;46:6593.Kang YK, Kim SM, Kim DY. J Am Chem Soc. 2010;132:11847.Cao WD, Liu XH, Wang WT, Lin LL, Feng XM. Org Lett. 2011;13:600.Haibach MC, Deb I, De CK, Seidel D. J Am Chem Soc. 2011;133:2100.Mori K, Ehara K, Kurihara K, Akiyama T. J Am Chem Soc. 2011;133:6166.Zhou GH, Liu F, Zhang JL. Chem Eur J. 2011;17:3101.He YP, Du YL, Luo SW, Gong LZ. Tetrahedron Lett. 2011;52:7064.Jurberg ID, Peng B, Woestefeld E, Wasserloos M, Maulide N. Angew Chem, Int Ed. 2012;51:1950.Sugiishi T, Nakamura H. J Am Chem Soc. 2012;134:2504.Wang Y, Chi Y, Zhang WX, Xi ZF. J Am Chem Soc. 2012;134:2926.Han YY, Han WY, Hou X, Zhang XM, Yuan WC. Org Lett. 2012;14:4054.Chen LJ, Zhang L, Lv J, Cheng JP, Luo SZ. Chem Eur J. 2012;18:8891.He YP, Wu H, Chen DF, Yu J, Gong LZ. Chem Eur J. 2013;19:5232.
-
-
- Zhang C, De CK, Mal R, Seidel D. J Am Chem Soc. 2008;130:416. - PubMed
- Deb I, Seidel D. Tetrahedron Lett. 2010;51:2945.
- Zhang C, Das D, Seidel D. Chem Sci. 2011;2:233.
- Deb I, Das D, Seidel D. Org Lett. 2011;13:812. - PubMed
- Zhang C, De CK, Seidel D. Org Synth. 2012;89:274.
- Ma L, Chen W, Seidel D. J Am Chem Soc. 2012;134:15305. - PubMed
- Das D, Sun AX, Seidel D. Angew Chem Int Ed. 2013;52:3765. - PMC - PubMed
- Dieckmann A, Richers MT, Platonova AY, Zhang C, Seidel D, Houk KN. J Org Chem. 2013;78:4132. - PMC - PubMed
- Richers MT, Deb I, Platonova AY, Zhang C, Seidel D. Synthesis. 2013;45:1730. - PMC - PubMed
- Das D, Seidel D. Org Lett. 2013;15:4358. - PMC - PubMed
-
-
Examples of related amine redox transformations: Oda M, Fukuchi Y, Ito S, Thanh NC, Kuroda S. Tetrahedron Lett. 2007;48:9159.Zheng L, Yang F, Dang Q, Bai X. Org Lett. 2008;10:889.Pahadi NK, Paley M, Jana R, Waetzig SR, Tunge JA. J Am Chem Soc. 2009;131:16626.Mao H, Xu R, Wan J, Jiang Z, Sun C, Pan Y. Chem Eur J. 2010;16:13352.Ghavtadze N, Narayan R, Wibbeling B, Wuerthwein EU. J Org Chem. 2011;76:5185.Xue XS, Yu A, Cai Y, Cheng JP. Org Lett. 2011;13:6054.Zheng Q-H, Meng W, Jiang G-J, Yu Z-X. Org Lett. 2013:15. doi: 10.1021/ol402517e.Lin W, Cao T, Fan W, Han Y, Kuang J, Luo H, Miao B, Tang X, Yu Q, Yuan W, Zhang J, Zhu C, Ma S. Angew Chem Int Ed. 2013:52. doi: 10.1002/ange.201308699.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

