Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.)
- PMID: 24328678
- PMCID: PMC4232904
- DOI: 10.1111/bjh.12708
Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.)
Abstract
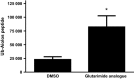
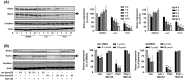
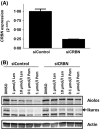
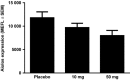
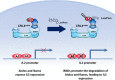
Cereblon (CRBN), the molecular target of lenalidomide and pomalidomide, is a substrate receptor of the cullin ring E3 ubiquitin ligase complex, CRL4(CRBN) . T cell co-stimulation by lenalidomide or pomalidomide is cereblon dependent: however, the CRL4(CRBN) substrates responsible for T cell co-stimulation have yet to be identified. Here we demonstrate that interaction of the transcription factors Ikaros (IKZF1, encoded by the IKZF1 gene) and Aiolos (IKZF3, encoded by the IKZF3 gene) with CRL4(CRBN) is induced by lenalidomide or pomalidomide. Each agent promotes Aiolos and Ikaros binding to CRL4(CRBN) with enhanced ubiquitination leading to cereblon-dependent proteosomal degradation in T lymphocytes. We confirm that Aiolos and Ikaros are transcriptional repressors of interleukin-2 expression. The findings link lenalidomide- or pomalidomide-induced degradation of these transcriptional suppressors to well documented T cell activation. Importantly, Aiolos could serve as a proximal pharmacodynamic marker for lenalidomide and pomalidomide, as healthy human subjects administered lenalidomide demonstrated Aiolos degradation in their peripheral T cells. In conclusion, we present a molecular model in which drug binding to cereblon results in the interaction of Ikaros and Aiolos to CRL4(CRBN) , leading to their ubiquitination, subsequent proteasomal degradation and T cell activation.
Keywords: Aiolos; Cereblon; Ikaros; lenalidomide; pomalidomide.
© 2013 The Authors. British Journal of Haematology published by John Wiley & Sons Ltd.
Figures




References
-
- Billot K, Parizot C, Arrouss I, Mazier D, Debre P, Rogner UC. Rebollo A. Differential Aiolos expression in human hematopoietic subpopulations. Leukemia Research. 2010;34:289–293. - PubMed
-
- Cai Q, Dierich A, Oulad-Abdelghani M, Chan S. Kastner P. Helios deficiency has minimal impact on T cell development and function. Journal of Immunology. 2009;183:2303–2311. - PubMed
-
- Chen N, Lau H, Kong L, Kumar G, Zeldis JB, Knight R. Laskin OL. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. Journal of Clinical Pharmacology. 2007;47:1466–1475. - PubMed
-
- Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI. Kaplan G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. Journal of Immunology. 1999;163:380–386. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

