Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress
- PMID: 24328731
- PMCID: PMC4174421
- DOI: 10.1089/ars.2013.5519
Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress
Abstract
Aims: Although uncoupling protein 2 (UCP2) negatively regulates intracellular reactive oxygen species (ROS) production and protects vascular function, its participation in vascular benefits of drugs used to treat cardiometabolic diseases is largely unknown. This study investigated whether UCP2 and associated oxidative stress reduction contribute to the improvement of endothelial function by a dipeptidyl peptidase-4 inhibitor, sitagliptin, in hypertension.
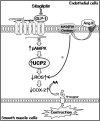
Results: Pharmacological inhibition of cyclooxygenase-2 (COX-2) but not COX-1 prevented endothelial dysfunction, and ROS scavengers reduced COX-2 mRNA and protein expression in spontaneously hypertensive rats (SHR) renal arteries. Angiotensin II (Ang II) evoked endothelium-dependent contractions (EDCs) in C57BL/6 and UCP2 knockout (UCP2KO) mouse aortae. Chronic sitagliptin administration attenuated EDCs in SHR arteries and Ang II-infused C57BL/6 mouse aortae and eliminated ROS overproduction in SHR arteries, which were reversed by glucagon-like peptide 1 receptor (GLP-1R) antagonist exendin 9-39, AMP-activated protein kinase (AMPK)α inhibitor compound C, and UCP2 inhibitor genipin. By contrast, sitagliptin unaffected EDCs in Ang II-infused UCP2KO mice. Sitagliptin increased AMPKα phosphorylation, upregulated UCP2, and downregulated COX-2 expression in arteries from SHR and Ang II-infused C57BL/6 mice. Importantly, exendin 9-39, compound C, and genipin reversed the inhibitory effect of GLP-1R agonist exendin-4 on Ang II-stimulated mitochondrial ROS rises in SHR endothelial cells. Moreover, exendin-4 improved the endothelial function of renal arteries from SHR and hypertensive patients.
Innovation: We elucidate for the first time that UCP2 serves as an important signal molecule in endothelial protection conferred by GLP-1-related agents. UCP2 could be a useful target in treating hypertension-related vascular events.
Conclusions: UCP2 inhibits oxidative stress and downregulates COX-2 expression through GLP-1/GLP-1R/AMPKα cascade.
Figures






References
-
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, and Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26: 435–439, 2000 - PubMed
-
- Bauer V. and Sotnikova R. Nitric oxide—the endothelium-derived relaxing factor and its role in endothelial functions. Gen Physiol Biophys 29: 319–340, 2010 - PubMed
-
- Brand MD. and Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2: 85–93, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

