Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease
- PMID: 24328763
- PMCID: PMC3976611
- DOI: 10.1111/bph.12516
Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease
Abstract
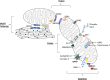
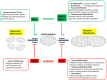
Mitochondria are no longer considered to be solely the static powerhouses of the cell. While they are undoubtedly essential to sustaining life and meeting the energy requirements of the cell through oxidative phosphorylation, they are now regarded as highly dynamic organelles with multiple functions, playing key roles in cell survival and death. In this review, we discuss the emerging role of mitochondrial fusion and fission proteins, as novel therapeutic targets for treating a wide range of cardiovascular diseases.
Keywords: Drp1; MFN1; MFN2; OPA1; mitochondrial dynamics; mitochondrial fission; mitochondrial fusion; mitochondrial morphology.
© 2013 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures
References
-
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

