Exploiting oxidative microenvironments in the body as triggers for drug delivery systems
- PMID: 24328819
- PMCID: PMC4098119
- DOI: 10.1089/ars.2013.5754
Exploiting oxidative microenvironments in the body as triggers for drug delivery systems
Abstract
Significance: Reactive oxygen species and reactive nitrogen species (ROS/RNS) play an important role in cell signaling pathways. However, the increased production of these species may disrupt cellular homeostasis, giving rise to pathological conditions. Biomaterials that are responsive to ROS/RNS can be strategically used to specifically release therapeutics and diagnostic agents to regions undergoing oxidative stress.
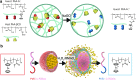
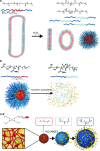
Recent advances: Many nanocarriers intended to exploit redox micro-environments as triggers for drug release, summarized and compared in this review, have recently been developed. We describe these carriers' chemical structures, strategies for payload protection and oxidation-selective release, and ROS/RNS sensitivity as tested in initial studies.
Critical issues: ROS/RNS are unstable, so reliable measures of their concentrations in various conditions are scarce. Combined with the dearth of materials shown to respond to physiologically relevant levels of ROS/RNS, evaluations of their true sensitivity are difficult.
Future directions: Oxidation-responsive nanocarriers developed thus far show tremendous potential for applicability in vivo; however, the sensitivity of these chemistries needs to be fine tuned to enable responses to physiological levels of ROS and RNS.
Figures






References
-
- Ahmed H. Principles and Reactions of Protein Extraction, Purification, and Characterization. Boca Raton, FL: Taylor & Francis, 2004
-
- Albrecht SC, Barata AG, Grosshans J, Teleman AA, and Dick TP. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab 14: 819–829, 2011 - PubMed
-
- Allen BL, Johnson JD, and Walker JP. Encapsulation and enzyme-mediated release of molecular cargo in polysulfide nanoparticles. ACS Nano 5: 5263–5272, 2011 - PubMed
-
- Amici A, Levine RL, Tsai L, and Stadtman ER. Conversion of amino-acid residues in proteins and amino-acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem 264: 3341–3346, 1989 - PubMed
-
- Antonenkov VD, Grunau S, Ohlmeier S, and Hiltunen JK. Peroxisomes are oxidative organelles. Antioxid Redox Signal 13: 525–537, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

