The inhibition of functional expression of calcium channels by prion protein demonstrates competition with α2δ for GPI-anchoring pathways
- PMID: 24329154
- PMCID: PMC3924758
- DOI: 10.1042/BJ20131405
The inhibition of functional expression of calcium channels by prion protein demonstrates competition with α2δ for GPI-anchoring pathways
Abstract
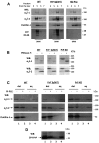
It has been shown recently that PrP (prion protein) and the calcium channel auxiliary α2δ subunits interact in neurons and expression systems [Senatore, Colleoni, Verderio, Restelli, Morini, Condliffe, Bertani, Mantovani, Canovi, Micotti, Forloni, Dolphin, Matteoli, Gobbi and Chiesa (2012) Neuron 74, 300-313]. In the present study we examined whether there was an effect of PrP on calcium currents. We have shown that when PrP is co-expressed with calcium channels formed from CaV2.1/β and α2δ-1 or α2δ-2, there is a consistent decrease in calcium current density. This reduction was absent when a PrP construct was used lacking its GPI (glycosylphosphatidylinositol) anchor. We have reported previously that α2δ subunits are able to form GPI-anchored proteins [Davies, Kadurin, Alvarez-Laviada, Douglas, Nieto-Rostro, Bauer, Pratt and Dolphin (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 1654-1659] and show further evidence in the present paper. We have characterized recently a C-terminally truncated α2δ-1 construct, α2δ-1ΔC, and found that, despite loss of its membrane anchor, it still shows a partial ability to increase calcium currents [Kadurin, Alvarez-Laviada, Ng, Walker-Gray, D'Arco, Fadel, Pratt and Dolphin (2012) J. Biol. Chem. 1287, 33554-33566]. We now find that PrP does not inhibit CaV2.1/β currents formed with α2δ-1ΔC, rather than α2δ-1. It is possible that PrP and α2δ-1 compete for GPI-anchor intermediates or trafficking pathways, or that interaction between PrP and α2δ-1 requires association in cholesterol-rich membrane microdomains. Our additional finding that CaV2.1/β1b/α2δ-1 currents were inhibited by GPI-GFP, but not cytosolic GFP, indicates that competition for limited GPI-anchor intermediates or trafficking pathways may be involved in PrP suppression of α2δ subunit function.
Figures




References
-
- Davies A., Hendrich J., Van Minh A. T., Wratten J., Douglas L., Dolphin A. C. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007;28:220–228. - PubMed
-
- Walker D., De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels. Trends Neurosci. 1998;21:148–154. - PubMed
-
- Dolphin A. C. β Subunits of voltage-gated calcium channels. J. Bioeng. Biomemb. 2003;35:599–620. - PubMed
-
- Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T. P., Campbell K. P. Calcium channel β-subunit binds to a conserved motif in the I–II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. - PubMed
-
- Leroy J., Richards M. S., Butcher A. J., Nieto-Rostro M., Pratt W. S., Davies A., Dolphin A. C. Interaction via a key tryptophan in the I–II linker of N-type calcium channels is required for beta1 but not for palmitoylated β2, implicating an additional binding site in the regulation of channel voltage-dependent properties. J. Neurosci. 2005;25:6984–6996. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

