The microbiome and regulation of mucosal immunity
- PMID: 24329495
- PMCID: PMC3992044
- DOI: 10.1111/imm.12231
The microbiome and regulation of mucosal immunity
Abstract
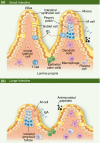
The gastrointestinal tract is a mucosal surface constantly exposed to foreign antigens and microbes, and is protected by a vast array of immunologically active structures and cells. Epithelial cells directly participate in immunological surveillance and direction of host responses in the gut and can express numerous pattern recognition receptors, including Toll-like receptor 5 (TLR5), TLR1, TLR2, TLR3, TLR9, and nucleotide oligomerization domain 2, as well as produce chemotactic factors for both myeloid and lymphoid cells following inflammatory stimulation. Within the epithelium and in the underlying lamina propria resides a population of innate lymphoid cells that, following stimulation, can become activated and produce effector cytokines and exert both protective and pathogenic roles during inflammation. Lamina propria dendritic cells play a large role in determining whether the response to a particular antigen will be inflammatory or anti-inflammatory. It is becoming clear that the composition and metabolic activity of the intestinal microbiome, as a whole community, exerts a profound influence on mucosal immune regulation. The microbiome produces short-chain fatty acids, polysaccharide A, α-galactosylceramide and tryptophan metabolites, which can induce interleukin-22, Reg3γ, IgA and interleukin-17 responses. However, much of what is known about microbiome-host immune interactions has come from the study of single bacterial members of the gastrointestinal microbiome and their impact on intestinal mucosal immunity. Additionally, evidence continues to accumulate that alterations of the intestinal microbiome can impact not only gastrointestinal immunity but also immune regulation at distal mucosal sites.
Keywords: immunity; inflammation; intestinal; microbiome; mucosal.
© 2013 John Wiley & Sons Ltd.
Figures

References
-
- Vereecke L, Beyaert R, Loo van G. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol Med. 2011;17:584–93. - PubMed
-
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–44. - PubMed
-
- Rio del ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol Rev. 2010;234:268–81. - PubMed
-
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

