An evolving story of angiotensin-II-forming pathways in rodents and humans
- PMID: 24329563
- PMCID: PMC4280795
- DOI: 10.1042/CS20130400
An evolving story of angiotensin-II-forming pathways in rodents and humans
Abstract
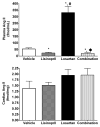
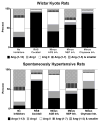
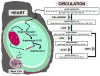
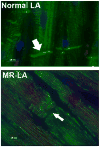
Lessons learned from the characterization of the biological roles of Ang-(1-7) [angiotensin-(1-7)] in opposing the vasoconstrictor, proliferative and prothrombotic actions of AngII (angiotensin II) created an underpinning for a more comprehensive exploration of the multiple pathways by which the RAS (renin-angiotensin system) of blood and tissues regulates homoeostasis and its altered state in disease processes. The present review summarizes the progress that has been made in the novel exploration of intermediate shorter forms of angiotensinogen through the characterization of the expression and functions of the dodecapeptide Ang-(1-12) [angiotensin-(1-12)] in the cardiac production of AngII. The studies reveal significant differences in humans compared with rodents regarding the enzymatic pathway by which Ang-(1-12) undergoes metabolism. Highlights of the research include the demonstration of chymase-directed formation of AngII from Ang-(1-12) in human left atrial myocytes and left ventricular tissue, the presence of robust expression of Ang-(1-12) and chymase in the atrial appendage of subjects with resistant atrial fibrillation, and the preliminary observation of significantly higher Ang-(1-12) expression in human left atrial appendages.
Figures


References
-
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. - PubMed
-
- Turner AJ, Tipnis SR, Guy JL, Rice G, Hooper NM. ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can J Physiol Pharmacol. 2002;80:346–353. - PubMed
-
- Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de BI, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

