Re-adapting T cells for cancer therapy: from mouse models to clinical trials
- PMID: 24329795
- PMCID: PMC4015625
- DOI: 10.1111/imr.12141
Re-adapting T cells for cancer therapy: from mouse models to clinical trials
Abstract
Adoptive T-cell therapy involves the isolation, expansion, and reinfusion of T lymphocytes with a defined specificity and function as a means to eradicate cancer. Our research has focused on specifying the requirements for tumor eradication with antigen-specific T cells and T cells transduced to express a defined T-cell receptor (TCR) in mouse models and then translating these strategies to clinical trials. Our design of T-cell-based therapy for cancer has reflected efforts to identify the obstacles that limit sustained effector T-cell activity in mice and humans, design approaches to enhance T-cell persistence, develop methods to increase TCR affinity/T-cell functional avidity, and pursue strategies to overcome tolerance and immunosuppression. With the advent of genetic engineering, a highly functional population of T cells can now be rapidly generated and tailored for the targeted malignancy. Preclinical studies in faithful and informative mouse models, in concert with knowledge gained from analyses of successes and limitations in clinical trials, are shaping how we continue to develop, refine, and broaden the applicability of this approach for cancer therapy.
Keywords: TCR gene therapy; adoptive T-cell therapy; clinical trials; high affinity; preclinical models.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
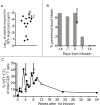
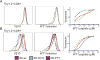
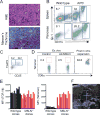
References
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. - PubMed
-
- Weiden PL, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. - PubMed
-
- Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. - PubMed
-
- Burstein NA, Law LW. Neonatal thymectomy and non-viral mammary tumours in mice. Nature. 1971;231:450–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

