Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus
- PMID: 24329967
- PMCID: PMC4065364
- DOI: 10.1111/jne.12125
Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus
Erratum in
- J Neuroendocrinol. 2014 Feb;26(2):121
Abstract
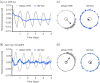
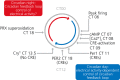
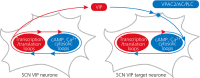
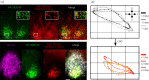
The suprachiasmatic nucleus (SCN) of the hypothalamus is the principal circadian pacemaker of the brain. It co-ordinates the daily rhythms of sleep and wakefulness, as well as physiology and behaviour, that set the tempo to our lives. Disturbance of this daily pattern, most acutely with jet-lag but more insidiously with rotational shift-work, can have severely deleterious effects for mental function and long-term health. The present review considers recent developments in our understanding of the properties of the SCN that make it a robust circadian time-keeper. It first focuses on the intracellular transcriptional/ translational feedback loops (TTFL) that constitute the cellular clockwork of the SCN neurone. Daily timing by these loops pivots around the negative regulation of the Period (Per) and Cryptochrome (Cry) genes by their protein products. The period of the circadian cycle is set by the relative stability of Per and Cry proteins, and this can be controlled by both genetic and pharmacological interventions. It then considers the function of these feedback loops in the context of cytosolic signalling by cAMP and intracellular calcium ([Ca(2+) ]i ), which are both outputs from, and inputs to, the TTFL, as well as the critical role of vasoactive intestinal peptide (VIP) signalling in synchronising cellular clocks across the SCN. Synchronisation by VIP in the SCN is paracrine, operating over an unconventionally long time frame (i.e. 24 h) and wide spatial domain, mediated via the cytosolic pathways upstream of the TTFL. Finally, we show how intersectional pharmacogenetics can be used to control G-protein-coupled signalling in individual SCN neurones, and how manipulation of Gq/[Ca(2+) ]i -signalling in VIP neurones can re-programme the circuit-level encoding of circadian time. Circadian pacemaking in the SCN therefore provides an unrivalled context in which to understand how a complex, adaptive behaviour can be organised by the dynamic activity of a relatively few gene products, operating in a clearly defined neuronal circuit, with both cell-autonomous and emergent, circuit-level properties.
Keywords: DREADD; VIP; paracrine; pharmacogenetic; sleep.
© 2014 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of The British Society for Neuroendocrinology.
Figures


References
-
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. - PubMed
-
- Akhtar RA, et al. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. - PubMed
-
- Reddy AB, et al. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–1115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

