The ROS-induced cytotoxicity of ascorbate is attenuated by hypoxia and HIF-1alpha in the NCI60 cancer cell lines
- PMID: 24330097
- PMCID: PMC3955158
- DOI: 10.1111/jcmm.12207
The ROS-induced cytotoxicity of ascorbate is attenuated by hypoxia and HIF-1alpha in the NCI60 cancer cell lines
Abstract
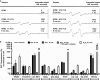
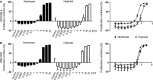
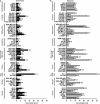
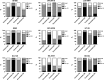
Intravenous application of high-dose ascorbate is used in complementary palliative medicine to treat cancer patients. Pharmacological doses of ascorbate in the mM range induce cytotoxicity in cancer cells mediated by reactive oxygen species (ROS), namely hydrogen peroxide and ascorbyl radicals. However, little is known about intrinsic or extrinsic factors modulating this ascorbate-mediated cytotoxicity. Under normoxia and hypoxia, ascorbate IC50 values were determined on the NCI60 cancer cells. The cell cycle, the influence of cobalt chloride-induced hypoxia-inducible factor-1α (HIF-1α) and the glucose transporter 1 (GLUT-1) expression (a pro-survival HIF-1α-downstream-target) were analysed after ascorbate exposure under normoxic and hypoxic conditions. The amount of ascorbyl radicals increased with rising serum concentrations. Hypoxia (0.1% O2 ) globally increased the IC50 of ascorbate in the 60 cancer cell lines from 4.5 ± 3.6 mM to 10.1 ± 5.9 mM (2.2-fold increase, P < 0.001, Mann-Whitney t-test), thus inducing cellular resistance towards ascorbate. This ascorbate resistance depended on HIF-1α-signalling, but did not correlate with cell line-specific expression of the ascorbate transporter GLUT-1. However, under normoxic and hypoxic conditions, ascorbate treatment at the individual IC50 reduced the expression of GLUT-1 in the cancer cells. Our data show a ROS-induced, HIF-1α- and O2 -dependent cytotoxicity of ascorbate on 60 different cancer cells. This suggests that for clinical application, cancer patients should additionally be oxygenized to increase the cytotoxic efficacy of ascorbate.
Keywords: GLUT-1; HIF-1α; ROS; ascorbate; cancer; hypoxia; therapy.
© 2013 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Padayatty SJ, Levine M. Reevaluation of ascorbate in cancer treatment: emerging evidence, open minds and serendipity. J Am Coll Nutr. 2000;19:423–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

