Cardiomyopathy reverses with recovery of liver injury, cholestasis and cholanemia in mouse model of biliary fibrosis
- PMID: 24330504
- PMCID: PMC4057995
- DOI: 10.1111/liv.12438
Cardiomyopathy reverses with recovery of liver injury, cholestasis and cholanemia in mouse model of biliary fibrosis
Abstract
Background: Triggers and exacerbants of cirrhotic cardiomyopathy (CC) are poorly understood, limiting treatment options in patients with chronic liver diseases. Liver transplantation alone reverses some features of CC, but the physiology behind this effect has never been studied.
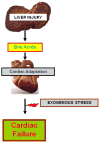
Aims: We aimed to determine whether reversal of liver injury and fibrosis in mouse affects cardiac parameters. The second aim was to determine whether cardiomyopathy can be induced by specifically increasing systemic bile acid (BA) levels.
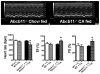
Methods: 6-8 week old male C57BL6J mice were fed either chow (n = 5) or 3,5-diethoxycarbonyl-1,4-dihydroxychollidine (DDC) (n = 10) for 3 weeks. At the end of 3 weeks, half the mice in the DDC fed group were randomized to chow (the reversed [REV] group). Serial ECHOs and electrocardiographic analysis was conducted weekly for 6 weeks followed by liver tissue and serum studies. Hearts were analysed for key components of function and cell signalling. Cardiac physiological and molecular parameters were similarly analysed in Abcb11(-/-) mice (n = 5/grp) fed 0.5% cholic acid supplemented diet for 1 week.
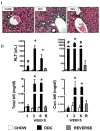
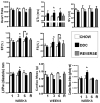
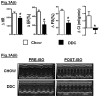
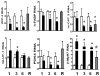
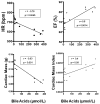
Results: Mice in the REV group showed normalization of biochemical markers of liver injury with resolution of electrocardiographic and ECHO aberrations. Catecholamine resistance seen in DDC group resolved in the REV group. Cardiac recovery was accompanied by normalization of cardiac troponin-T2 as well as resolution of cardiac stress response at RNA level. Cardiovascular physiological and molecular parameters correlated with degree of cholanemia. Cardiomyopathy was reproduced in cholanemic BA fed Abcb11(-/-) mice.
Conclusions: Cardiomyopathy resolves with resolution of liver injury, is associated with cholanaemia, and can be induced by BA feeding.
Keywords: bile acid-myocardial interaction; cardiac adaptation; cholanaemia; hepatic - cardiopathy; liver injury.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures






Similar articles
-
Protective role of cardiac-specific overexpression of caveolin-3 in cirrhotic cardiomyopathy.Am J Physiol Gastrointest Liver Physiol. 2020 Mar 1;318(3):G531-G541. doi: 10.1152/ajpgi.00346.2019. Epub 2020 Jan 21. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 31961720 Free PMC article.
-
Da-Huang-Xiao-Shi decoction protects against3, 5-diethoxycarbonyl-1,4-dihydroxychollidine-induced chronic cholestasis by upregulating bile acid metabolic enzymes and efflux transporters.J Ethnopharmacol. 2021 Apr 6;269:113706. doi: 10.1016/j.jep.2020.113706. Epub 2020 Dec 18. J Ethnopharmacol. 2021. PMID: 33346024
-
Impaired bile acid handling and aggravated liver injury in mice expressing a hepatocyte-specific RXRα variant lacking the DNA-binding domain.J Hepatol. 2014 Feb;60(2):362-9. doi: 10.1016/j.jhep.2013.09.026. Epub 2013 Oct 10. J Hepatol. 2014. PMID: 24120911 Free PMC article.
-
Loss of hepatocyte Usp53 protects mice from a form of xenobiotic-induced liver injury.Biochim Biophys Acta Mol Basis Dis. 2025 Mar;1871(3):167624. doi: 10.1016/j.bbadis.2024.167624. Epub 2024 Dec 19. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 39705897 Review.
-
Animal models of biliary tract injury.Curr Opin Gastroenterol. 2012 May;28(3):239-43. doi: 10.1097/MOG.0b013e32835264d9. Curr Opin Gastroenterol. 2012. PMID: 22450892 Review.
Cited by
-
Protective role of cardiac-specific overexpression of caveolin-3 in cirrhotic cardiomyopathy.Am J Physiol Gastrointest Liver Physiol. 2020 Mar 1;318(3):G531-G541. doi: 10.1152/ajpgi.00346.2019. Epub 2020 Jan 21. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 31961720 Free PMC article.
-
Cardiac changes in pediatric liver transplant recipients: are they truly irreversible?Hepatol Int. 2016 May;10(3):390-3. doi: 10.1007/s12072-016-9708-7. Epub 2016 Feb 16. Hepatol Int. 2016. PMID: 26884376 No abstract available.
-
Dysregulated Calcium Handling in Cirrhotic Cardiomyopathy.Biomedicines. 2023 Jul 4;11(7):1895. doi: 10.3390/biomedicines11071895. Biomedicines. 2023. PMID: 37509534 Free PMC article. Review.
-
Redox-Dependent Effects in the Physiopathological Role of Bile Acids.Oxid Med Cell Longev. 2021 Sep 4;2021:4847941. doi: 10.1155/2021/4847941. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34527174 Free PMC article. Review.
-
The Role of Bile Acids in Cardiovascular Diseases: from Mechanisms to Clinical Implications.Aging Dis. 2023 Apr 1;14(2):261-282. doi: 10.14336/AD.2022.0817. eCollection 2023 Apr 1. Aging Dis. 2023. PMID: 37008052 Free PMC article.
References
-
- Alqahtani SA, Fouad TR, Lee SS. Cirrhotic cardiomyopathy. Semin Liver Dis. 2008 Feb;28(1):59–69. - PubMed
-
- Moller S, Hove JD, Dixen U, Bendtsen F. New insights into cirrhotic cardiomyopathy. Int J Cardiol. 2012 Oct 4; - PubMed
-
- Zardi EM, Abbate A, Zardi DM, Dobrina A, Margiotta D, Van Tassel BW, et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010 Aug 10;56(7):539–549. - PubMed
-
- Arikan C, Kilic M, Tumgor G, Levent E, Yuksekkaya HA, Yagci RV, et al. Impact of liver transplantation on rate-corrected QT interval and myocardial function in children with chronic liver disease*. Pediatr Transplant. 2009 May;13(3):300–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

