Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know?
- PMID: 24331192
- PMCID: PMC3880205
- DOI: 10.1053/j.seminoncol.2013.09.006
Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know?
Abstract
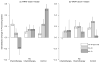
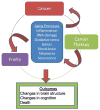
There is a fairly consistent, albeit non-universal body of research documenting cognitive declines after cancer and its treatments. While few of these studies have included subjects aged 65 years and older, it is logical to expect that older patients are at risk of cognitive decline. Here, we use breast cancer as an exemplar disease for inquiry into the intersection of aging and cognitive effects of cancer and its therapies. There are a striking number of common underlying potential biological risks and pathways for the development of cancer, cancer-related cognitive declines, and aging processes, including the development of a frail phenotype. Candidate shared pathways include changes in hormonal milieu, inflammation, oxidative stress, DNA damage and compromised DNA repair, genetic susceptibility, decreased brain blood flow or disruption of the blood-brain barrier, direct neurotoxicity, decreased telomere length, and cell senescence. There also are similar structure and functional changes seen in brain imaging studies of cancer patients and those seen with "normal" aging and Alzheimer's disease. Disentangling the role of these overlapping processes is difficult since they require aged animal models and large samples of older human subjects. From what we do know, frailty and its low cognitive reserve seem to be a clinically useful marker of risk for cognitive decline after cancer and its treatments. This and other results from this review suggest the value of geriatric assessments to identify older patients at the highest risk of cognitive decline. Further research is needed to understand the interactions between aging, genetic predisposition, lifestyle factors, and frailty phenotypes to best identify the subgroups of older patients at greatest risk for decline and to develop behavioral and pharmacological interventions targeting this group. We recommend that basic science and population trials be developed specifically for older hosts with intermediate endpoints of relevance to this group, including cognitive function and trajectories of frailty. Clinicians and their older patients can advance the field by active encouragement of and participation in research designed to improve the care and outcomes of the growing population of older cancer patients.
© 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS; [Accessed May 1, 2012]. ( www.seer.cancer.gov) SEER*Stat Database: Mortality-All COD, Aggregated with state, total U.S. (1969–2009)<Katrina/Rita Population Adjustment>-linked to county attributes-total U.S., 1969–2010 counties.
-
- United States Census Bureau, U.S. Department of Commerce, Economics and Statistics Administration. [Accessed 2008];Population by age, sex, race and Hispanic and Latino origin for the United States. 2011 http://www.census.gov/population/www.cen2000/briefs/phc-t9/tables/tab01.pdf.
-
- Silberfarb PM. Chemotherapy and cognitive defects in cancer patients. Annu Rev Med. 1983;34:35–46. - PubMed
-
- Eberhardt B, Dilger S, Musial F, Wedding U, Weiss T, Miltner WH. Short-term monitoring of cognitive functions before and during the first course of treatment. J Cancer Res Clin Oncol. 2006;132:234–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA137788/CA/NCI NIH HHS/United States
- R01 CA127617/CA/NCI NIH HHS/United States
- R01CA127617/CA/NCI NIH HHS/United States
- R01CA129769/CA/NCI NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- U24AG021886/AG/NIA NIH HHS/United States
- C06 RR020128/RR/NCRR NIH HHS/United States
- S10RR027710/RR/NCRR NIH HHS/United States
- U10 CA084131/CA/NCI NIH HHS/United States
- R01 CA101318/CA/NCI NIH HHS/United States
- K05CA096940/CA/NCI NIH HHS/United States
- U54132378/PHS HHS/United States
- R01AG019771/AG/NIA NIH HHS/United States
- P30CA51008/CA/NCI NIH HHS/United States
- R01 AG019771/AG/NIA NIH HHS/United States
- S10 RR027710/RR/NCRR NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- R25 CA117865/CA/NCI NIH HHS/United States
- R01 CA087845/CA/NCI NIH HHS/United States
- P30 CA082709/CA/NCI NIH HHS/United States
- R01CA101318/CA/NCI NIH HHS/United States
- U54 CA132378/CA/NCI NIH HHS/United States
- F30AG039959/AG/NIA NIH HHS/United States
- P30 CA051008/CA/NCI NIH HHS/United States
- U54RR025761/RR/NCRR NIH HHS/United States
- P30AG010133/AG/NIA NIH HHS/United States
- R01 CA129769/CA/NCI NIH HHS/United States
- F30 AG039959/AG/NIA NIH HHS/United States
- K05 CA096940/CA/NCI NIH HHS/United States
- C06RR020128/RR/NCRR NIH HHS/United States
- U10CA84131/CA/NCI NIH HHS/United States
- P30-AG13846/AG/NIA NIH HHS/United States
- R25CA117865/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

