Anti-human protein S antibody induces tissue factor expression through a direct interaction with platelet phosphofructokinase
- PMID: 24331211
- PMCID: PMC3947162
- DOI: 10.1016/j.thromres.2013.11.009
Anti-human protein S antibody induces tissue factor expression through a direct interaction with platelet phosphofructokinase
Abstract
Introduction: Autoantibodies including anti-human protein S antibody (anti-hPS Ab) and anti-human protein C antibody (anti-hPC Ab) can be detected in patients with autoimmune diseases with hypercoagulability. The objective of the present study was to determine the effects and molecular pathways of these autoantibodies on tissue factor (TF) expression in human coronary artery endothelial cells (HCAECs).
Materials and methods: HCAECs were treated with anti-hPS Ab or anti-hPC Ab for 3 hours. TF expression was measured by real-time PCR and Western blot. TF-mediated procoagulant activity was determined by a commercial kit. MAPK phosphorylation was analyzed by Bio-Plex luminex immunoassay and Western blot. The potential proteins interacting with anti-hPS Ab were studied by immunoprecipitation, mass spectrometry and in vitro pull-down assay.
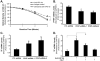
Results: Anti-hPS Ab, but not anti-hPC Ab, specifically induced TF expression and TF-mediated procoagulant activity in HCAECs in a concentration-dependent manner. This effect was confirmed in human umbilical endothelial cells (HUVECs). ERK1/2 phosphorylation was induced by anti-hPS Ab treatment, while inhibition of ERK1/2 by U0216 partially blocked anti-hPS Ab-induced TF upregulation (P<0.05). In addition, anti-hPS Ab specifically cross-interacted with platelet phosphofructokinase (PFKP) in HCAECs. Anti-hPS Ab was able to directly inhibit PFKP activities in HCAECs. Furthermore, silencing of PFKP by PFKP shRNA resulted in TF upregulation in HCAECs, while activation of PFKP by fructose-6-phosphate partially blocked the effect of anti-hPS Ab on TF upregulation (P<0.05).
Conclusions: Anti-hPS Ab induces TF expression through a direct interaction with PFKP and ERK1/2 activation in HCAECs. Anti-hPS Ab may directly contribute to vascular thrombosis in the patient with autoimmune disorders.
Keywords: Anti-human protein S antibody; Autoantibody; ERK1/2; Endothelial cell; Platelet phosphofructokinase; Tissue factor.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
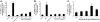
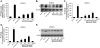
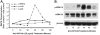
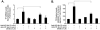
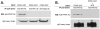
References
-
- Walker FJ. Protein S and the regulation of activated protein C. Semin Thromb Hemost. 1984;10:131–8. - PubMed
-
- Bereczky Z, Kovács KB, Muszbek L. Protein C and protein S deficiencies: similarities and differences between two brothers playing in the same game. Clin Chem Lab Med. 2010;48(Suppl 1):S53–66. - PubMed
-
- D'Angelo A, Della Valle P, Crippa L, Pattarini E, Grimaldi LM, Vigano D'Angelo S. Brief report: autoimmune protein S deficiency in a boy with severe thromboembolic disease. N Engl J Med. 1993;328:1753–7. - PubMed
-
- Walker FJ. Regulation of activated protein C by protein S. The role of phospholipid in factor Va inactivation. J Biol Chem. 1981;256:11128–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

