Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models
- PMID: 24332043
- PMCID: PMC3917315
- DOI: 10.1016/j.ccr.2013.10.013
Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models
Abstract
High-grade serous ovarian carcinoma presents significant clinical and therapeutic challenges. Although the traditional model of carcinogenesis has focused on the ovary as a tumor initiation site, recent studies suggest that there may be additional sites of origin outside the ovary, namely the secretory cells of the fallopian tube. Our study demonstrates that high-grade serous tumors can originate in fallopian tubal secretory epithelial cells and also establishes serous tubal intraepithelial carcinoma as the precursor lesion to high-grade serous ovarian and peritoneal carcinomas in animal models targeting the Brca, Tp53, and Pten genes. These findings offer an avenue to address clinically important questions that are critical for cancer prevention and early detection in women carrying BRCA1 and BRCA2 mutations.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

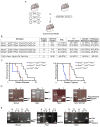




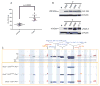
Comment in
-
Ovarian cancer: A better mimic.Nat Rev Cancer. 2014 Feb;14(2):74-5. doi: 10.1038/nrc3674. Nat Rev Cancer. 2014. PMID: 24457414 No abstract available.
References
-
- Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–2663. - PMC - PubMed
-
- Bast RC., Jr Status of tumor markers in ovarian cancer screening. J Clin Oncol. 2003;21:200s–205s. - PubMed
-
- Bertucci MC, Mitchell CA. Phosphoinositide 3-kinase and INPP4B in human breast cancer. Ann NY Acad Sci. 2013;1280:1–5. - PubMed
-
- Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB, McDonald JF. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104:331–337. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

