Molecular mechanisms underlying the enhanced functions of three-dimensional hepatocyte aggregates
- PMID: 24332390
- PMCID: PMC4012541
- DOI: 10.1016/j.biomaterials.2013.11.063
Molecular mechanisms underlying the enhanced functions of three-dimensional hepatocyte aggregates
Abstract
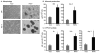
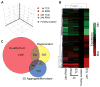
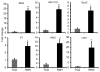
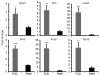
Three-dimensional (3D) culture of hepatocytes leads to improved and prolonged synthetic and metabolic functions, but the underlying molecular mechanisms are unknown. In order to investigate the role of 3D cell-cell interactions in maintaining hepatocyte differentiated functions ex vivo, primary mouse hepatocytes were cultured either as monolayers on tissue culture dishes (TCD) or as 3D aggregates in rotating wall vessel (RWV) bioreactors. Global gene expression analyses revealed that genes upregulated in 3D culture were distinct from those upregulated during liver development and liver regeneration. Instead, they represented a diverse array of hepatocyte-specific functional genes with significant over-representation of hepatocyte nuclear factor 4α (Hnf4a) binding sites in their promoters. Expression of Hnf4a and many of its downstream target genes were significantly increased in RWV cultures as compared to TCD. Conversely, there was concomitant suppression of mesenchymal and cytoskeletal genes in RWV cultures that were induced in TCDs. These findings illustrate the importance of 3D cell-cell interactions in maintaining fundamental molecular pathways of hepatocyte function and serve as a basis for rational design of biomaterials that aim to optimize hepatocyte functions ex vivo for biomedical applications.
Keywords: Bioreactor; Gene expression; Hepatocyte; Microarray; Spheroid; Three-dimensional cell culture.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
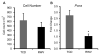
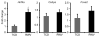
Similar articles
-
Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes.Tissue Eng Part A. 2009 Mar;15(3):559-67. doi: 10.1089/ten.tea.2007.0434. Tissue Eng Part A. 2009. PMID: 18724832 Free PMC article.
-
Reconstitution of hepatic tissue architectures from fetal liver cells obtained from a three-dimensional culture with a rotating wall vessel bioreactor.J Biosci Bioeng. 2011 Jun;111(6):711-8. doi: 10.1016/j.jbiosc.2011.01.019. Epub 2011 Mar 12. J Biosci Bioeng. 2011. PMID: 21402492
-
Extending hepatocyte functionality for drug-testing applications using high-viscosity alginate-encapsulated three-dimensional cultures in bioreactors.Tissue Eng Part C Methods. 2010 Dec;16(6):1223-32. doi: 10.1089/ten.TEC.2009.0784. Epub 2010 Apr 6. Tissue Eng Part C Methods. 2010. PMID: 20184401
-
Primary Hepatocyte Isolation and Cultures: Technical Aspects, Challenges and Advancements.Bioengineering (Basel). 2023 Jan 18;10(2):131. doi: 10.3390/bioengineering10020131. Bioengineering (Basel). 2023. PMID: 36829625 Free PMC article. Review.
-
[Development of novel culture system for regulation of hepatocyte function].Yakugaku Zasshi. 2010 Apr;130(4):537-43. doi: 10.1248/yakushi.130.537. Yakugaku Zasshi. 2010. PMID: 20371999 Review. Japanese.
Cited by
-
Changes in Gene Expression and Estrogen Receptor Cistrome in Mouse Liver Upon Acute E2 Treatment.Mol Endocrinol. 2016 Jul;30(7):709-32. doi: 10.1210/me.2015-1311. Epub 2016 May 10. Mol Endocrinol. 2016. PMID: 27164166 Free PMC article.
-
Direct lysis of 3D cell cultures for RT-qPCR gene expression quantification.Sci Rep. 2023 Jan 27;13(1):1520. doi: 10.1038/s41598-023-28844-1. Sci Rep. 2023. PMID: 36707637 Free PMC article.
-
The impact of simulated and real microgravity on bone cells and mesenchymal stem cells.Biomed Res Int. 2014;2014:928507. doi: 10.1155/2014/928507. Epub 2014 Jul 10. Biomed Res Int. 2014. PMID: 25110709 Free PMC article. Review.
-
Self-Assembled Matrigel-Free iPSC-Derived Liver Organoids Demonstrate Wide-Ranging Highly Differentiated Liver Functions.Stem Cells. 2023 Mar 2;41(2):126-139. doi: 10.1093/stmcls/sxac090. Stem Cells. 2023. PMID: 36573434 Free PMC article.
-
Advances in space microbiology.iScience. 2021 Apr 3;24(5):102395. doi: 10.1016/j.isci.2021.102395. eCollection 2021 May 21. iScience. 2021. PMID: 33997680 Free PMC article. Review.
References
-
- Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–45. - PubMed
-
- Brown LA, Arterburn LM, Miller AP, Cowger NL, Hartley SM, Andrews A, et al. Maintenance of liver functions in rat hepatocytes cultured as spheroids in a rotating wall vessel. In Vitro Cell Dev Biol Anim. 2003;39:13–20. - PubMed
-
- Ji R, Zhang N, You N, Li Q, Liu W, Jiang N, et al. The differentiation of MSCs into functional hepatocyte-like cells in a liver biomatrix scaffold and their transplantation into liver-fibrotic mice. Biomaterials. 2012;33:8995–9008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

