An ecological network of polysaccharide utilization among human intestinal symbionts
- PMID: 24332541
- PMCID: PMC3924574
- DOI: 10.1016/j.cub.2013.10.077
An ecological network of polysaccharide utilization among human intestinal symbionts
Abstract
Background: The human intestine is colonized with trillions of microorganisms important to health and disease. There has been an intensive effort to catalog the species and genetic content of this microbial ecosystem. However, little is known of the ecological interactions between these microbes, a prerequisite to understanding the dynamics and stability of this host-associated microbial community. Here we perform a systematic investigation of public goods-based syntrophic interactions among the abundant human gut bacteria, the Bacteroidales.
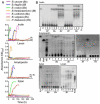
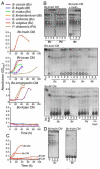
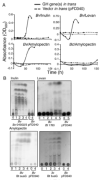
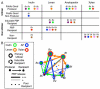
Results: We find evidence for a rich interaction network based on the breakdown and use of polysaccharides. Species that utilize a particular polysaccharide (producers) liberate polysaccharide breakdown products (PBPs) that are consumed by other species unable to grow on the polysaccharide alone (recipients). Cross-species gene addition experiments demonstrate that recipients can grow on a polysaccharide if the producer-derived glycoside hydrolase, responsible for PBP generation, is provided. These producer-derived glycoside hydrolases are public goods transported extracellularly in outer membrane vesicles allowing for the creation of PBP and concomitant recipient growth spatially distant from the producer. Recipients can exploit these ecological interactions and conditionally outgrow producers. Finally, we show that these public goods-based interactions occur among Bacteroidales species coresident within a natural human intestinal community.
Conclusions: This study examines public goods-based syntrophic interactions between bacterial members of the human gut microbial ecosystem. This polysaccharide-based network likely represents foundational relationships creating organized ecological units within the intestinal microbiota, knowledge of which can be applied to impact human health.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Evidence of extensive DNA transfer between bacteroidales species within the human gut.mBio. 2014 Jun 17;5(3):e01305-14. doi: 10.1128/mBio.01305-14. mBio. 2014. PMID: 24939888 Free PMC article.
-
Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla.Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):5859-64. doi: 10.1073/pnas.0901529106. Epub 2009 Mar 24. Proc Natl Acad Sci U S A. 2009. PMID: 19321416 Free PMC article.
-
A refined palate: bacterial consumption of host glycans in the gut.Glycobiology. 2013 Sep;23(9):1038-46. doi: 10.1093/glycob/cwt040. Epub 2013 May 28. Glycobiology. 2013. PMID: 23720460 Free PMC article. Review.
-
Cultivable, Host-Specific Bacteroidetes Symbionts Exhibit Diverse Polysaccharolytic Strategies.Appl Environ Microbiol. 2020 Apr 1;86(8):e00091-20. doi: 10.1128/AEM.00091-20. Print 2020 Apr 1. Appl Environ Microbiol. 2020. PMID: 32060023 Free PMC article.
-
PUL-Mediated Plant Cell Wall Polysaccharide Utilization in the Gut Bacteroidetes.Int J Mol Sci. 2021 Mar 17;22(6):3077. doi: 10.3390/ijms22063077. Int J Mol Sci. 2021. PMID: 33802923 Free PMC article. Review.
Cited by
-
Improving microbial fitness in the mammalian gut by in vivo temporal functional metagenomics.Mol Syst Biol. 2015 Mar;11(3):788. doi: 10.15252/msb.20145866. Mol Syst Biol. 2015. PMID: 26148351 Free PMC article.
-
Characterization and Spatial Mapping of the Human Gut Metasecretome.mSystems. 2022 Dec 20;7(6):e0071722. doi: 10.1128/msystems.00717-22. Epub 2022 Dec 5. mSystems. 2022. PMID: 36468852 Free PMC article.
-
Antibiotic Korormicin A Kills Bacteria by Producing Reactive Oxygen Species.J Bacteriol. 2019 May 8;201(11):e00718-18. doi: 10.1128/JB.00718-18. Print 2019 Jun 1. J Bacteriol. 2019. PMID: 30858300 Free PMC article.
-
Lipidomics Analysis of Outer Membrane Vesicles and Elucidation of the Inositol Phosphoceramide Biosynthetic Pathway in Bacteroides thetaiotaomicron.Microbiol Spectr. 2022 Feb 23;10(1):e0063421. doi: 10.1128/spectrum.00634-21. Epub 2022 Jan 26. Microbiol Spectr. 2022. PMID: 35080445 Free PMC article.
-
Microbial transcriptional responses to host diet maintain gut microbiome homeostasis in the American cockroach.bioRxiv [Preprint]. 2024 Nov 19:2024.10.31.621369. doi: 10.1101/2024.10.31.621369. bioRxiv. 2024. PMID: 39554183 Free PMC article. Preprint.
References
-
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

