A self-sequestered calmodulin-like Ca²⁺ sensor of mitochondrial SCaMC carrier and its implication to Ca²⁺-dependent ATP-Mg/P(i) transport
- PMID: 24332718
- PMCID: PMC3946054
- DOI: 10.1016/j.str.2013.10.018
A self-sequestered calmodulin-like Ca²⁺ sensor of mitochondrial SCaMC carrier and its implication to Ca²⁺-dependent ATP-Mg/P(i) transport
Abstract
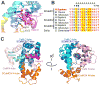
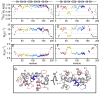
The mitochondrial carriers play essential roles in energy metabolism. The short Ca²⁺-binding mitochondrial carrier (SCaMC) transports ATP-Mg in exchange for Pi and is important for activities that depend on adenine nucleotides. SCaMC adopts, in addition to the transmembrane domain (TMD) that transports solutes, an extramembrane N-terminal domain (NTD) that regulates solute transport in a Ca²⁺-dependent manner. Crystal structure of the Ca²⁺-bound NTD reveals a compact architecture in which the functional EF hands are sequestered by an endogenous helical segment. Nuclear magnetic resonance (NMR) relaxation rates indicated that removal of Ca²⁺ from NTD results in a major conformational switch from the rigid and compact Ca²⁺-bound state to the dynamic and loose apo state. Finally, we showed using surface plasmon resonance and NMR titration experiments that free apo NTDs could specifically interact with liposome-incorporated TMD, but that Ca²⁺ binding drastically weakened the interaction. Our results together provide a molecular explanation for Ca²⁺-dependent ATP-Mg flux in mitochondria.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Molecular Basis of MgATP Selectivity of the Mitochondrial SCaMC Carrier.Structure. 2015 Aug 4;23(8):1394-1403. doi: 10.1016/j.str.2015.06.004. Epub 2015 Jul 9. Structure. 2015. PMID: 26165595 Free PMC article.
-
Purification, crystallization and preliminary X-ray diffraction of the N-terminal calmodulin-like domain of the human mitochondrial ATP-Mg/Pi carrier SCaMC1.Acta Crystallogr F Struct Biol Commun. 2014 Jan;70(Pt 1):68-71. doi: 10.1107/S2053230X1303241X. Epub 2013 Dec 24. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 24419621 Free PMC article.
-
Identification of a novel human subfamily of mitochondrial carriers with calcium-binding domains.J Biol Chem. 2004 Jun 4;279(23):24701-13. doi: 10.1074/jbc.M401417200. Epub 2004 Mar 30. J Biol Chem. 2004. PMID: 15054102
-
Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs).Biochim Biophys Acta. 2016 Aug;1857(8):1158-1166. doi: 10.1016/j.bbabio.2016.04.003. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060251 Review.
-
Calcium-regulated mitochondrial ATP-Mg/Pi carriers evolved from a fusion of an EF-hand regulatory domain with a mitochondrial ADP/ATP carrier-like domain.IUBMB Life. 2018 Dec;70(12):1222-1232. doi: 10.1002/iub.1931. Epub 2018 Oct 3. IUBMB Life. 2018. PMID: 30281880 Free PMC article. Review.
Cited by
-
Studying Peptide-Metal Ion Complex Structures by Solution-State NMR.Int J Mol Sci. 2022 Dec 15;23(24):15957. doi: 10.3390/ijms232415957. Int J Mol Sci. 2022. PMID: 36555599 Free PMC article. Review.
-
The SLC25 Carrier Family: Important Transport Proteins in Mitochondrial Physiology and Pathology.Physiology (Bethesda). 2020 Sep 1;35(5):302-327. doi: 10.1152/physiol.00009.2020. Physiology (Bethesda). 2020. PMID: 32783608 Free PMC article. Review.
-
Molecular Basis of MgATP Selectivity of the Mitochondrial SCaMC Carrier.Structure. 2015 Aug 4;23(8):1394-1403. doi: 10.1016/j.str.2015.06.004. Epub 2015 Jul 9. Structure. 2015. PMID: 26165595 Free PMC article.
-
Calcium-induced conformational changes in the regulatory domain of the human mitochondrial ATP-Mg/Pi carrier.Biochim Biophys Acta. 2015 Oct;1847(10):1245-53. doi: 10.1016/j.bbabio.2015.07.002. Epub 2015 Jul 9. Biochim Biophys Acta. 2015. PMID: 26164100 Free PMC article.
-
Calcium regulation of the human mitochondrial ATP-Mg/Pi carrier SLC25A24 uses a locking pin mechanism.Sci Rep. 2017 Mar 28;7:45383. doi: 10.1038/srep45383. Sci Rep. 2017. PMID: 28350015 Free PMC article.
References
-
- Amigo I, Traba J, Gonzalez-Barroso MM, Rueda CB, Fernandez M, Rial E, Sanchez A, Satrustegui J, Del Arco A. Glucagon Regulation of Oxidative Phosphorylation Requires an Increase in Matrix Adenine Nucleotide Content through Ca2+ Activation of the Mitochondrial ATP-Mg/Pi Carrier SCaMC-3. The Journal of biological chemistry. 2013;288:7791–7802. - PMC - PubMed
-
- Anunciado-Koza RP, Zhang J, Ukropec J, Bajpeyi S, Koza RA, Rogers RC, Cefalu WT, Mynatt RL, Kozak LP. Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice. The Journal of biological chemistry. 2011;286:11659–11671. - PMC - PubMed
-
- Barbato G, Ikura M, Kay LE, Pastor RW, Bax A. Backbone dynamics of calmodulin studied by 15N relaxation using inverse detected two-dimensional NMR spectroscopy: the central helix is flexible. Biochemistry. 1992;31:5269–5278. - PubMed
-
- Bassi MT, Manzoni M, Bresciani R, Pizzo MT, Della Monica A, Barlati S, Monti E, Borsani G. Cellular expression and alternative splicing of SLC25A23, a member of the mitochondrial Ca2+-dependent solute carrier gene family. Gene. 2005;345:173–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

