A view to a kill: the bacterial type VI secretion system
- PMID: 24332978
- PMCID: PMC3936019
- DOI: 10.1016/j.chom.2013.11.008
A view to a kill: the bacterial type VI secretion system
Abstract
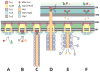
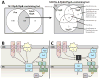
The bacterial type VI secretion system (T6SS) is an organelle that is structurally and mechanistically analogous to an intracellular membrane-attached contractile phage tail. Recent studies determined that a rapid conformational change in the structure of a sheath protein complex propels T6SS spike and tube components along with antibacterial and antieukaryotic effectors out of predatory T6SS(+) cells and into prey cells. The contracted organelle is then recycled in an ATP-dependent process. T6SS is regulated at transcriptional and posttranslational levels, the latter involving detection of membrane perturbation in some species. In addition to directly targeting eukaryotic cells, the T6SS can also target other bacteria coinfecting a mammalian host, highlighting the importance of the T6SS not only for bacterial survival in environmental ecosystems, but also in the context of infection and disease. This review highlights these and other advances in our understanding of the structure, mechanical function, assembly, and regulation of the T6SS.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Architecture and assembly of the Type VI secretion system.Biochim Biophys Acta. 2014 Aug;1843(8):1664-73. doi: 10.1016/j.bbamcr.2014.03.018. Epub 2014 Mar 26. Biochim Biophys Acta. 2014. PMID: 24681160 Review.
-
PAAR-repeat proteins sharpen and diversify the type VI secretion system spike.Nature. 2013 Aug 15;500(7462):350-353. doi: 10.1038/nature12453. Epub 2013 Aug 7. Nature. 2013. PMID: 23925114 Free PMC article.
-
Biochemical analysis of TssK, a core component of the bacterial Type VI secretion system, reveals distinct oligomeric states of TssK and identifies a TssK-TssFG subcomplex.Biochem J. 2014 Jul 15;461(2):291-304. doi: 10.1042/BJ20131426. Biochem J. 2014. PMID: 24779861 Free PMC article.
-
Type VI secretion system: secretion by a contractile nanomachine.Philos Trans R Soc Lond B Biol Sci. 2015 Oct 5;370(1679):20150021. doi: 10.1098/rstb.2015.0021. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26370934 Free PMC article. Review.
-
Marker for type VI secretion system effectors.Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):9271-6. doi: 10.1073/pnas.1406110111. Epub 2014 Jun 9. Proc Natl Acad Sci U S A. 2014. PMID: 24927539 Free PMC article.
Cited by
-
Comparative genomics provides insights into the potential biocontrol mechanism of two Lysobacter enzymogenes strains with distinct antagonistic activities.Front Microbiol. 2022 Aug 11;13:966986. doi: 10.3389/fmicb.2022.966986. eCollection 2022. Front Microbiol. 2022. PMID: 36033849 Free PMC article.
-
Spatial structure, cooperation and competition in biofilms.Nat Rev Microbiol. 2016 Sep;14(9):589-600. doi: 10.1038/nrmicro.2016.84. Epub 2016 Jul 25. Nat Rev Microbiol. 2016. PMID: 27452230 Review.
-
Roles of Hcp2, a Hallmark of T6SS2 in Motility, Adhesive Capacity, and Pathogenicity of Vibrio alginolyticus.Microorganisms. 2023 Nov 30;11(12):2893. doi: 10.3390/microorganisms11122893. Microorganisms. 2023. PMID: 38138037 Free PMC article.
-
Type IV secretion system effector sabotages multiple defense systems in a competing bacterium.ISME J. 2024 Jan 8;18(1):wrae121. doi: 10.1093/ismejo/wrae121. ISME J. 2024. PMID: 38959853 Free PMC article.
-
Spatial Organization of Expanding Bacterial Colonies Is Affected by Contact-Dependent Growth Inhibition.Curr Biol. 2019 Nov 4;29(21):3622-3634.e5. doi: 10.1016/j.cub.2019.08.074. Epub 2019 Oct 17. Curr Biol. 2019. PMID: 31630946 Free PMC article.
References
-
- Arisaka F, Kanamaru S, Leiman P, Rossmann MG. The tail lysozyme complex of bacteriophage T4. Int J Biochem Cell Biol. 2003;35:16–21. - PubMed
-
- Aschtgen MS, Thomas MS, Cascales E. Anchoring the type VI secretion system to the peptidoglycan: TssL, TagL, TagP... what else? Virulence. 2010;1:535–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

