FGF signaling is required for brain left-right asymmetry and brain midline formation
- PMID: 24333178
- PMCID: PMC3970204
- DOI: 10.1016/j.ydbio.2013.11.020
FGF signaling is required for brain left-right asymmetry and brain midline formation
Abstract
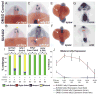
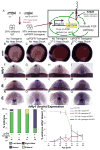
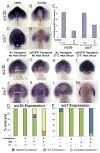
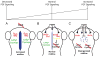
Early disruption of FGF signaling alters left-right (LR) asymmetry throughout the embryo. Here we uncover a role for FGF signaling that specifically disrupts brain asymmetry, independent of normal lateral plate mesoderm (LPM) asymmetry. When FGF signaling is inhibited during mid-somitogenesis, asymmetrically expressed LPM markers southpaw and lefty2 are not affected. However, asymmetrically expressed brain markers lefty1 and cyclops become bilateral. We show that FGF signaling controls expression of six3b and six7, two transcription factors required for repression of asymmetric lefty1 in the brain. We found that Z0-1, atypical PKC (aPKC) and β-catenin protein distribution revealed a midline structure in the forebrain that is dependent on a balance of FGF signaling. Ectopic activation of FGF signaling leads to overexpression of six3b, loss of organized midline adherins junctions and bilateral loss of lefty1 expression. Reducing FGF signaling leads to a reduction in six3b and six7 expression, an increase in cell boundary formation in the brain midline, and bilateral expression of lefty1. Together, these results suggest a novel role for FGF signaling in the brain to control LR asymmetry, six transcription factor expressions, and a midline barrier structure.
Keywords: Brain asymmetry; FGF signaling; Sine occulis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Two additional midline barriers function with midline lefty1 expression to maintain asymmetric Nodal signaling during left-right axis specification in zebrafish.Development. 2011 Oct;138(20):4405-10. doi: 10.1242/dev.071092. Development. 2011. PMID: 21937597 Free PMC article.
-
Antagonistic interactions in the zebrafish midline prior to the emergence of asymmetric gene expression are important for left-right patterning.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 19;371(1710):20150402. doi: 10.1098/rstb.2015.0402. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27821532 Free PMC article.
-
Initiation and propagation of posterior to anterior (PA) waves in zebrafish left-right development.Dev Dyn. 2008 Dec;237(12):3640-7. doi: 10.1002/dvdy.21771. Dev Dyn. 2008. PMID: 18985756 Free PMC article.
-
Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry.Development. 2000 Aug;127(16):3567-79. doi: 10.1242/dev.127.16.3567. Development. 2000. PMID: 10903181
-
Left-right asymmetry in zebrafish.Cell Mol Life Sci. 2012 Sep;69(18):3069-77. doi: 10.1007/s00018-012-0985-6. Epub 2012 Apr 19. Cell Mol Life Sci. 2012. PMID: 22527718 Free PMC article. Review.
Cited by
-
LEFTY2/endometrial bleeding-associated factor up-regulates Na+ Coupled Glucose Transporter SGLT1 expression and Glycogen Accumulation in Endometrial Cancer Cells.PLoS One. 2020 Apr 1;15(4):e0230044. doi: 10.1371/journal.pone.0230044. eCollection 2020. PLoS One. 2020. PMID: 32236143 Free PMC article.
-
Swimming through asymmetry: zebrafish as a model for brain and behavior lateralization.Front Behav Neurosci. 2025 Jan 20;19:1527572. doi: 10.3389/fnbeh.2025.1527572. eCollection 2025. Front Behav Neurosci. 2025. PMID: 39906337 Free PMC article. Review.
-
Development and connectivity of the habenular nuclei.Semin Cell Dev Biol. 2018 Jun;78:107-115. doi: 10.1016/j.semcdb.2017.10.007. Epub 2017 Nov 6. Semin Cell Dev Biol. 2018. PMID: 29107475 Free PMC article. Review.
-
Logics and properties of a genetic regulatory program that drives embryonic muscle development in an echinoderm.Elife. 2015 Jul 28;4:e07343. doi: 10.7554/eLife.07343. Elife. 2015. PMID: 26218224 Free PMC article.
-
Brain and Behavioral Asymmetry: A Lesson From Fish.Front Neuroanat. 2020 Mar 26;14:11. doi: 10.3389/fnana.2020.00011. eCollection 2020. Front Neuroanat. 2020. PMID: 32273841 Free PMC article. Review.
References
-
- Concha ML, Signore IA, Colombo A. Mechanisms of directional asymmetry in the zebrafish epithalamus. Semin Cell Dev Biol. 2009;20:498–509. - PubMed
-
- Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000;28:399–409. - PubMed
-
- Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development (Cambridge, England) 2000;127:5101–5112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

