A cellular model of amyloid precursor protein processing and amyloid-β peptide production
- PMID: 24333289
- PMCID: PMC3931259
- DOI: 10.1016/j.jneumeth.2013.11.024
A cellular model of amyloid precursor protein processing and amyloid-β peptide production
Abstract
Background: A hallmark pathologic feature of Alzheimer's disease (AD) is accumulation of neuritic senile plaques in the brain parenchyma. Neurotoxic plaque cores are composed predominantly of amyloid-β (Aβ) peptides of 40 and 42 amino acids in length, formed by sequential cleavage of amyloid precursor protein (APP) by β-, and γ-secretases. There is a great interest in approaches to modulate Aβ peptide production and develop therapeutic interventions to reduce Aβ levels to halt or slow the progression of neurodegeneration.
New method: We characterized and present the BE(2)-M17 human neuroblastoma cell line as a novel in vitro model of the APP-cleavage cascade to support future (1) functional studies of molecular regulators in Aβ production, and (2) high-throughput screening assays of new pharmacotherapeutics.
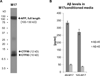
Results: In BE(2)-M17 cells, both RNA (i.e., RT-PCR, RNA sequencing) and protein analyses (i.e., Western blots, ELISA), show endogenous expression of critical components of the amyloidogenic pathway, APP-cleavage intermediates CTF83 and CTF99, and final cleavage products Aβ40 and Aβ42. We further report effects of retinoic acid-mediated differentiation on morphology and gene expression in this cell line.
Comparison with existing method(s): In contrast to primary isolates or other cell lines reported in current literature, BE(2)-M17 not only sustains baseline expression of the full contingent of APP-processing components, but also remains stably adherent during culture, facilitating experimental manipulations.
Conclusions: Our evidence supports the use of BE(2)-M17 as a novel, human, cell-based model of the APP processing pathway that offers a potential streamlined approach to dissect molecular functions of endogenous regulatory pathways, and perform mechanistic studies to identify modulators of Aβ production.
Keywords: Alzheimer's; Amyloid-beta; BE(2)-M17; In vitro; Secretase; TNF.
Copyright © 2013 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Andrau D, Dumanchin-Njock C, Aryal E, Vizzavona J, Farzan M, Boisbrun M, Fulcrand P, Hernandez J-F, Martinez J, Lefranc-Jullien S, et al. BACE1- and BACE2-expressing Human Cells. The Journal of Biological Chemistry. 2003;278(28):25859–25866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

