Circadian organization of the mammalian retina: from gene regulation to physiology and diseases
- PMID: 24333669
- PMCID: PMC3927986
- DOI: 10.1016/j.preteyeres.2013.12.001
Circadian organization of the mammalian retina: from gene regulation to physiology and diseases
Abstract
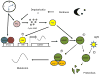
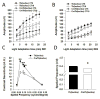
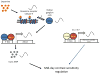
The retinal circadian system represents a unique structure. It contains a complete circadian system and thus the retina represents an ideal model to study fundamental questions of how neural circadian systems are organized and what signaling pathways are used to maintain synchrony of the different structures in the system. In addition, several studies have shown that multiple sites within the retina are capable of generating circadian oscillations. The strength of circadian clock gene expression and the emphasis of rhythmic expression are divergent across vertebrate retinas, with photoreceptors as the primary locus of rhythm generation in amphibians, while in mammals clock activity is most robust in the inner nuclear layer. Melatonin and dopamine serve as signaling molecules to entrain circadian rhythms in the retina and also in other ocular structures. Recent studies have also suggested GABA as an important component of the system that regulates retinal circadian rhythms. These transmitter-driven influences on clock molecules apparently reinforce the autonomous transcription-translation cycling of clock genes. The molecular organization of the retinal clock is similar to what has been reported for the SCN although inter-neural communication among retinal neurons that form the circadian network is apparently weaker than those present in the SCN, and it is more sensitive to genetic disruption than the central brain clock. The melatonin-dopamine system is the signaling pathway that allows the retinal circadian clock to reconfigure retinal circuits to enhance light-adapted cone-mediated visual function during the day and dark-adapted rod-mediated visual signaling at night. Additionally, the retinal circadian clock also controls circadian rhythms in disk shedding and phagocytosis, and possibly intraocular pressure. Emerging experimental data also indicate that circadian clock is also implicated in the pathogenesis of eye disease and compelling experimental data indicate that dysfunction of the retinal circadian system negatively impacts the retina and possibly the cornea and the lens.
Keywords: Circadian; Cornea; Dopamine; Electroretinogram; Melatonin; Photoreceptors; Retina; Retinal pigment epithelium.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

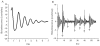
Similar articles
-
The Retina and Other Light-sensitive Ocular Clocks.J Biol Rhythms. 2016 Jun;31(3):223-43. doi: 10.1177/0748730416642657. Epub 2016 Apr 19. J Biol Rhythms. 2016. PMID: 27095816 Free PMC article. Review.
-
An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA.PLoS Biol. 2008 Oct 14;6(10):e249. doi: 10.1371/journal.pbio.0060249. PLoS Biol. 2008. PMID: 18959477 Free PMC article.
-
Endogenous functioning and light response of the retinal clock in vertebrates.Prog Brain Res. 2022;273(1):49-69. doi: 10.1016/bs.pbr.2022.04.011. Epub 2022 Jul 18. Prog Brain Res. 2022. PMID: 35940724 Review.
-
Circadian clock organization in the retina: From clock components to rod and cone pathways and visual function.Prog Retin Eye Res. 2023 May;94:101119. doi: 10.1016/j.preteyeres.2022.101119. Epub 2022 Dec 8. Prog Retin Eye Res. 2023. PMID: 36503722 Free PMC article. Review.
-
The Retinal Circadian Clock and Photoreceptor Viability.Adv Exp Med Biol. 2018;1074:345-350. doi: 10.1007/978-3-319-75402-4_42. Adv Exp Med Biol. 2018. PMID: 29721962 Free PMC article. Review.
Cited by
-
Rhodopsin-mediated light-off-induced protein kinase A activation in mouse rod photoreceptor cells.Proc Natl Acad Sci U S A. 2020 Oct 27;117(43):26996-27003. doi: 10.1073/pnas.2009164117. Epub 2020 Oct 12. Proc Natl Acad Sci U S A. 2020. PMID: 33046651 Free PMC article.
-
Melatonin Entrains PER2::LUC Bioluminescence Circadian Rhythm in the Mouse Cornea.Invest Ophthalmol Vis Sci. 2015 Jul;56(8):4753-8. doi: 10.1167/iovs.15-17124. Invest Ophthalmol Vis Sci. 2015. PMID: 26207312 Free PMC article.
-
Mammalian retinal Müller cells have circadian clock function.Mol Vis. 2016 Mar 26;22:275-83. eCollection 2016. Mol Vis. 2016. PMID: 27081298 Free PMC article.
-
Dim Light at Night and Constant Darkness: Two Frequently Used Lighting Conditions That Jeopardize the Health and Well-being of Laboratory Rodents.Front Neurol. 2018 Aug 2;9:609. doi: 10.3389/fneur.2018.00609. eCollection 2018. Front Neurol. 2018. PMID: 30116218 Free PMC article. Review.
-
Altered ocular parameters from circadian clock gene disruptions.PLoS One. 2019 Jun 18;14(6):e0217111. doi: 10.1371/journal.pone.0217111. eCollection 2019. PLoS One. 2019. PMID: 31211778 Free PMC article.
References
-
- Aihara M, Lindsey JD, Weinreb RN. Twenty-four-hour pattern of mouse intraocular pressure. Exp Eye Res. 2003;77:681–686. - PubMed
-
- Ait-Hmyed O, Felder-Schmittbuhl MP, Garcia-Garrido M, Beck S, Seide C, et al. Mice lacking Period 1 and Period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur J Neurosci. 2013:371048–60. - PubMed
-
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16:100–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY004864/EY/NEI NIH HHS/United States
- P30-EY008126/EY/NEI NIH HHS/United States
- P30 EY006360/EY/NEI NIH HHS/United States
- G12 RR003034/RR/NCRR NIH HHS/United States
- R01 EY022216/EY/NEI NIH HHS/United States
- U54 NS083932/NS/NINDS NIH HHS/United States
- R01 EY015815/EY/NEI NIH HHS/United States
- EY015815/EY/NEI NIH HHS/United States
- T32 EY007092/EY/NEI NIH HHS/United States
- S21 MD000101/MD/NIMHD NIH HHS/United States
- EY022216/EY/NEI NIH HHS/United States
- G12-RR03034/RR/NCRR NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

