A mitochondrial location for haemoglobins--dynamic distribution in ageing and Parkinson's disease
- PMID: 24333691
- PMCID: PMC3969298
- DOI: 10.1016/j.mito.2013.12.001
A mitochondrial location for haemoglobins--dynamic distribution in ageing and Parkinson's disease
Abstract
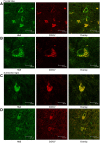
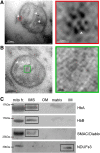
Haemoglobins are iron-containing proteins that transport oxygen in the blood of most vertebrates. The mitochondrion is the cellular organelle which consumes oxygen in order to synthesise ATP. Mitochondrial dysfunction is implicated in neurodegeneration and ageing. We find that α and β haemoglobin (Hba and Hbb) proteins are altered in their distribution in mitochondrial fractions from degenerating brain. We demonstrate that both Hba and Hbb are co-localised with the mitochondrion in mammalian brain. The precise localisation of the Hbs is within the inner membrane space and associated with inner mitochondrial membrane. Relative mitochondrial to cytoplasmic ratios of Hba and Hbb show changing distributions of these proteins during the process of neurodegeneration in the pcd(5j) mouse brain. A significant difference in mitochondrial Hba and Hbb content in the mitochondrial fraction is seen at 31 days after birth, this corresponds to a stage when dynamic neuronal loss is measured to be greatest in the Purkinje Cell Degeneration mouse. We also report changes in mitochondrial Hba and Hbb levels in ageing brain and muscle. Significant differences in mitochondrial Hba and Hbb can be seen when comparing aged brain to muscle, suggesting tissue specific functions of these proteins in the mitochondrion. In muscle there are significant differences between Hba levels in old and young mitochondria. To understand whether the changes detected in mitochondrial Hbs are of clinical significance, we examined Parkinson's disease brain, immunohistochemistry studies suggest that cell bodies in the substantia nigra accumulate mitochondrial Hb. However, western blotting of mitochondrial fractions from PD and control brains indicates significantly less Hb in PD brain mitochondria. One explanation could be a specific loss of cells containing mitochondria loaded with Hb proteins. Our study opens the door to an examination of the role of Hb function, within the context of the mitochondrion-in health and disease.
Keywords: Ageing; Haemoglobin; Mitochondria; Neurodegeneration; Parkinson's Disease.
Copyright © 2013 The authors. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Neuronal hemoglobin in mitochondria is reduced by forming a complex with α-synuclein in aging monkey brains.Oncotarget. 2016 Feb 16;7(7):7441-54. doi: 10.18632/oncotarget.7046. Oncotarget. 2016. PMID: 26824991 Free PMC article.
-
Analysis of Mitochondrial haemoglobin in Parkinson's disease brain.Mitochondrion. 2016 Jul;29:45-52. doi: 10.1016/j.mito.2016.05.001. Epub 2016 May 12. Mitochondrion. 2016. PMID: 27181046 Free PMC article.
-
Neurons express hemoglobin alpha- and beta-chains in rat and human brains.J Comp Neurol. 2009 Aug 10;515(5):538-47. doi: 10.1002/cne.22062. J Comp Neurol. 2009. PMID: 19479992 Free PMC article.
-
Ubiquinone (coenzyme q10) and mitochondria in oxidative stress of parkinson's disease.Biol Signals Recept. 2001 May-Aug;10(3-4):224-53. doi: 10.1159/000046889. Biol Signals Recept. 2001. PMID: 11351130 Review.
-
Emerging concepts of mitochondrial dysfunction in Parkinson's disease progression: Pathogenic and therapeutic implications.Mitochondrion. 2020 Jan;50:25-34. doi: 10.1016/j.mito.2019.09.010. Epub 2019 Oct 22. Mitochondrion. 2020. PMID: 31654753 Review.
Cited by
-
Mitochondrial proteomic profiling reveals increased carbonic anhydrase II in aging and neurodegeneration.Aging (Albany NY). 2016 Oct 10;8(10):2425-2436. doi: 10.18632/aging.101064. Aging (Albany NY). 2016. PMID: 27743511 Free PMC article.
-
Neuronal hemoglobin in mitochondria is reduced by forming a complex with α-synuclein in aging monkey brains.Oncotarget. 2016 Feb 16;7(7):7441-54. doi: 10.18632/oncotarget.7046. Oncotarget. 2016. PMID: 26824991 Free PMC article.
-
Whole Blood Gene Expression Profiling in Preclinical and Clinical Cattle Infected with Atypical Bovine Spongiform Encephalopathy.PLoS One. 2016 Apr 13;11(4):e0153425. doi: 10.1371/journal.pone.0153425. eCollection 2016. PLoS One. 2016. PMID: 27073865 Free PMC article.
-
Network Analysis of a Membrane-Enriched Brain Proteome across Stages of Alzheimer's Disease.Proteomes. 2019 Aug 27;7(3):30. doi: 10.3390/proteomes7030030. Proteomes. 2019. PMID: 31461916 Free PMC article.
-
Proteomic analysis of the ATP synthase interactome in notothenioids highlights a pathway that inhibits ceruloplasmin production.Am J Physiol Regul Integr Comp Physiol. 2022 Aug 1;323(2):R181-R192. doi: 10.1152/ajpregu.00069.2022. Epub 2022 May 31. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 35639858 Free PMC article.
References
-
- Alexa A., Rahnenführer J., Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics. 2006;22:1600–1607. - PubMed
-
- Barbaric I., Miller G., Dear T.N. Appearances can be deceiving: phenotypes of knockout mice. Brief. Funct. Genomic Proteomic. 2007;6:91–103. - PubMed
-
- Bénard G., Massa F., Puente N., Lourenço J., Bellocchio L. Mitochondrial CB₁ receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012;15:558–564. - PubMed
-
- Chakrabarti L., Neal J.T., Miles M., Martinez R.A., Smith A.C. The Purkinje cell degeneration 5 J mutation is a single amino acid insertion that destabilizes Nna1 protein. Mamm. Genome. 2006;17:103–110. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

