Microparticles produced by the hydrogel template method for sustained drug delivery
- PMID: 24333903
- PMCID: PMC3920451
- DOI: 10.1016/j.ijpharm.2013.11.058
Microparticles produced by the hydrogel template method for sustained drug delivery
Abstract
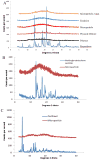
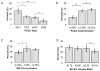
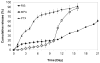
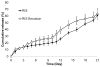
Polymeric microparticles have been used widely for sustained drug delivery. Current methods of microparticle production can be improved by making homogeneous particles in size and shape, increasing the drug loading, and controlling the initial burst release. In the current study, the hydrogel template method was used to produce homogeneous poly(lactide-co-glycolide) (PLGA) microparticles and to examine formulation and process-related parameters. Poly(vinyl alcohol) (PVA) was used to make hydrogel templates. The parameters examined include PVA molecular weight, type of PLGA (as characterized by lactide content, inherent viscosity), polymer concentration, drug concentration and composition of solvent system. Three model compounds studied were risperidone, methylprednisolone acetate and paclitaxel. The ability of the hydrogel template method to produce microparticles with good conformity to template was dependent on molecular weight of PVA and viscosity of the PLGA solution. Drug loading and encapsulation efficiency were found to be influenced by PLGA lactide content, polymer concentration and composition of the solvent system. The drug loading and encapsulation efficiency were 28.7% and 82% for risperidone, 31.5% and 90% for methylprednisolone acetate, and 32.2% and 92% for paclitaxel, respectively. For all three drugs, release was sustained for weeks, and the in vitro release profile of risperidone was comparable to that of microparticles prepared using the conventional emulsion method. The hydrogel template method provides a new approach of manipulating microparticles.
Keywords: Controlled release; Methylprednisolone acetate; PLGA microparticle; PVA template method; Paclitaxel; Risperidone.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures






Similar articles
-
Control of encapsulation efficiency and morphology of poly(lactide-co-glycolide) microparticles with a diafiltration-driven solvent extraction process.Eur J Pharm Biopharm. 2024 Nov;204:114515. doi: 10.1016/j.ejpb.2024.114515. Epub 2024 Sep 24. Eur J Pharm Biopharm. 2024. PMID: 39326801
-
Enhanced encapsulation and bioavailability of breviscapine in PLGA microparticles by nanocrystal and water-soluble polymer template techniques.Eur J Pharm Biopharm. 2017 Jun;115:177-185. doi: 10.1016/j.ejpb.2017.02.021. Epub 2017 Mar 2. Eur J Pharm Biopharm. 2017. PMID: 28263795
-
Effects of formulation parameters on encapsulation efficiency and release behavior of risperidone poly(D,L-lactide-co-glycolide) microsphere.Chem Pharm Bull (Tokyo). 2009 Nov;57(11):1251-6. doi: 10.1248/cpb.57.1251. Chem Pharm Bull (Tokyo). 2009. PMID: 19881277
-
Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives.J Control Release. 2008 Feb 11;125(3):193-209. doi: 10.1016/j.jconrel.2007.09.013. Epub 2007 Oct 22. J Control Release. 2008. PMID: 18083265 Review.
-
Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: State-of-the-art and challenges.Int J Pharm. 2016 Feb 29;499(1-2):358-367. doi: 10.1016/j.ijpharm.2016.01.020. Epub 2016 Jan 12. Int J Pharm. 2016. PMID: 26795193 Review.
Cited by
-
A reproducible accelerated in vitro release testing method for PLGA microspheres.Int J Pharm. 2016 Feb 10;498(1-2):274-82. doi: 10.1016/j.ijpharm.2015.12.031. Epub 2015 Dec 15. Int J Pharm. 2016. PMID: 26705156 Free PMC article.
-
PLA micro- and nano-particles.Adv Drug Deliv Rev. 2016 Dec 15;107:176-191. doi: 10.1016/j.addr.2016.05.020. Epub 2016 Jun 1. Adv Drug Deliv Rev. 2016. PMID: 27262925 Free PMC article. Review.
-
pH Responsive Hydrogels for the Delivery of Capecitabine: Development, Optimization and Pharmacokinetic Studies.Gels. 2022 Nov 28;8(12):775. doi: 10.3390/gels8120775. Gels. 2022. PMID: 36547299 Free PMC article.
-
Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading.Front Pharmacol. 2016 Jun 28;7:185. doi: 10.3389/fphar.2016.00185. eCollection 2016. Front Pharmacol. 2016. PMID: 27445821 Free PMC article. Review.
-
PLGA sustained-release microspheres loaded with an insoluble small-molecule drug: microfluidic-based preparation, optimization, characterization, and evaluation in vitro and in vivo.Drug Deliv. 2022 Dec;29(1):1437-1446. doi: 10.1080/10717544.2022.2072413. Drug Deliv. 2022. PMID: 35532150 Free PMC article.
References
-
- Tran VT, Benoit JP, Venier-Julienne MC. Why and how to prepare biodegradable, monodispersed, polymeric microparticles in the field of pharmacy? International Journal of Pharmaceutics. 2011;407:1–11. - PubMed
-
- Wei Y, Wang YX, Kang AJ, Wang W, Ho SV, Gao JF, Ma GH, Su ZG. A Novel Sustained-Release Formulation of Recombinant Human Growth Hormone and Its Pharmacokinetic, Pharmacodynamic and Safety Profiles. Molecular pharmaceutics. 2012;9:2039–2048. - PubMed
-
- Yeoand Y, Park KN. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Archives of Pharmacal Research. 2004;27:1–12. - PubMed
-
- Su ZX, Shi YN, Teng LS, Li X, Wang LX, Meng QF, Teng LR, Li YX. Biodegradable poly(D, L-lactide-co-glycolide) (PLGA) microspheres for sustained release of risperidone: Zero-order release formulation. Pharmaceutical Development and Technology. 2011;16:377–384. - PubMed
-
- Amann LC, Gandal MJ, Lin R, Liang YL, Siegel SJ. In Vitro-In Vivo Correlations of Scalable PLGA-Risperidone Implants for the Treatment of Schizophrenia. Pharmaceutical Research. 2010;27:1730–1737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

