β-arrestin1-biased β1-adrenergic receptor signaling regulates microRNA processing
- PMID: 24334028
- PMCID: PMC3955054
- DOI: 10.1161/CIRCRESAHA.114.302766
β-arrestin1-biased β1-adrenergic receptor signaling regulates microRNA processing
Abstract
Rationale: MicroRNAs (miRs) are small, noncoding RNAs that function to post-transcriptionally regulate gene expression. First transcribed as long primary miR transcripts (pri-miRs), they are enzymatically processed in the nucleus by Drosha into hairpin intermediate miRs (pre-miRs) and further processed in the cytoplasm by Dicer into mature miRs where they regulate cellular processes after activation by a variety of signals such as those stimulated by β-adrenergic receptors (βARs). Initially discovered to desensitize βAR signaling, β-arrestins are now appreciated to transduce multiple effector pathways independent of G-protein-mediated second messenger accumulation, a concept known as biased signaling. We previously showed that the β-arrestin-biased βAR agonist, carvedilol, activates cellular pathways in the heart.
Objective: Here, we tested whether carvedilol could activate β-arrestin-mediated miR maturation, thereby providing a novel potential mechanism for its cardioprotective effects.
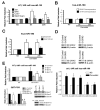
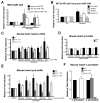
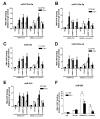
Methods and results: In human cells and mouse hearts, carvedilol upregulates a subset of mature and pre-miRs, but not their pri-miRs, in β1AR-, G-protein-coupled receptor kinase 5/6-, and β-arrestin1-dependent manner. Mechanistically, β-arrestin1 regulates miR processing by forming a nuclear complex with hnRNPA1 and Drosha on pri-miRs.
Conclusions: Our findings indicate a novel function for β1AR-mediated β-arrestin1 signaling activated by carvedilol in miR biogenesis, which may be linked, in part, to its mechanism for cell survival.
Keywords: carvedilol; heart diseases.
Figures




Comment in
-
Embracing bias: β1-adrenergic receptor-biased ligands and nuclear miRNA processing.Circ Res. 2014 Feb 28;114(5):742-5. doi: 10.1161/CIRCRESAHA.114.303237. Circ Res. 2014. PMID: 24577958 No abstract available.
Similar articles
-
β-arrestin-biased agonism of β-adrenergic receptor regulates Dicer-mediated microRNA maturation to promote cardioprotective signaling.J Mol Cell Cardiol. 2018 May;118:225-236. doi: 10.1016/j.yjmcc.2018.04.001. Epub 2018 Apr 6. J Mol Cell Cardiol. 2018. PMID: 29627294 Free PMC article.
-
Gαi is required for carvedilol-induced β1 adrenergic receptor β-arrestin biased signaling.Nat Commun. 2017 Nov 22;8(1):1706. doi: 10.1038/s41467-017-01855-z. Nat Commun. 2017. PMID: 29167435 Free PMC article.
-
Carvedilol-responsive microRNAs, miR-199a-3p and -214 protect cardiomyocytes from simulated ischemia-reperfusion injury.Am J Physiol Heart Circ Physiol. 2016 Aug 1;311(2):H371-83. doi: 10.1152/ajpheart.00807.2015. Epub 2016 Jun 10. Am J Physiol Heart Circ Physiol. 2016. PMID: 27288437 Free PMC article.
-
β-arrestin-mediated signaling improves the efficacy of therapeutics.J Pharmacol Sci. 2012;118(4):408-12. doi: 10.1254/jphs.11r10cp. Epub 2012 Mar 23. J Pharmacol Sci. 2012. PMID: 22447307 Review.
-
Beta-arrestin-mediated signaling in the heart.Circ J. 2008 Nov;72(11):1725-9. doi: 10.1253/circj.cj-08-0734. Epub 2008 Oct 7. Circ J. 2008. PMID: 18838825 Free PMC article. Review.
Cited by
-
Pathophysiology and pharmacology of G protein-coupled receptors in the heart.Cardiovasc Res. 2023 May 22;119(5):1117-1129. doi: 10.1093/cvr/cvac171. Cardiovasc Res. 2023. PMID: 36534965 Free PMC article. Review.
-
Identification of gene signatures regulated by carvedilol in mouse heart.Physiol Genomics. 2015 Sep;47(9):376-85. doi: 10.1152/physiolgenomics.00028.2015. Epub 2015 Jul 7. Physiol Genomics. 2015. PMID: 26152686 Free PMC article.
-
Knockdown of Plakophilin 2 Downregulates miR-184 Through CpG Hypermethylation and Suppression of the E2F1 Pathway and Leads to Enhanced Adipogenesis In Vitro.Circ Res. 2016 Sep 2;119(6):731-50. doi: 10.1161/CIRCRESAHA.116.308422. Epub 2016 Jul 28. Circ Res. 2016. PMID: 27470638 Free PMC article.
-
MicroRNA-34c-5p provokes isoprenaline-induced cardiac hypertrophy by modulating autophagy via targeting ATG4B.Acta Pharm Sin B. 2022 May;12(5):2374-2390. doi: 10.1016/j.apsb.2021.09.020. Epub 2021 Sep 25. Acta Pharm Sin B. 2022. PMID: 35646533 Free PMC article.
-
Endothelin-1/endothelin A receptor-mediated biased signaling is a new player in modulating human ovarian cancer cell tumorigenesis.Cell Signal. 2014 Dec;26(12):2885-95. doi: 10.1016/j.cellsig.2014.08.024. Epub 2014 Sep 3. Cell Signal. 2014. PMID: 25194819 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

