Combination therapy of hydroxycarbamide with anagrelide in patients with essential thrombocythemia in the evaluation of Xagrid(R) efficacy and long-term safety study
- PMID: 24334294
- PMCID: PMC3971078
- DOI: 10.3324/haematol.2012.083097
Combination therapy of hydroxycarbamide with anagrelide in patients with essential thrombocythemia in the evaluation of Xagrid(R) efficacy and long-term safety study
Abstract
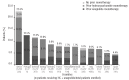
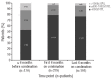
Available information is limited regarding the use of cytoreductive combination therapy in high-risk patients with essential thrombocythemia. This analysis aims to evaluate the clinical relevance and patterns of cytoreductive combination treatment in European high-risk patients with essential thrombocythemia in the Evaluation of Xagrid(®) Efficacy and Long-term Safety study. Of 3643 patients, 347 (9.5%) received combination therapy. Data were recorded at each 6-month update. Of 347 patients who received combination therapy, 304 (87.6%) received hydroxycarbamide + anagrelide. Monotherapies received before this combination were hydroxycarbamide (n=167, 54.9%) and anagrelide (n=123, 40.5%). Median weekly doses of hydroxycarbamide and anagrelide were: 7000 and 10.5 mg when used as prior monotherapy; 3500 and 7.0 mg when used as add-on treatment. Overall, median platelet counts were 581 × 10(9)/L and 411 × 10(9)/L before and after starting hydroxycarbamide + anagrelide, respectively. In patients with paired data (n=153), the number of patients with platelet counts less than 400 × 10(9)/L increased from 33 (21.6%) to 74 (48.4%; P<0.0001), and with platelet counts less than 600 × 10(9)/L, from 82 (53.6%) to 132 (86.3%; P<0.0001). Hydroxycarbamide + anagrelide was discontinued in 158 patients: 76 (48.1%) stopped hydroxycarbamide, 59 (37.3%) stopped anagrelide, 19 (12.0%) stopped both and 4 (2.5%) had another therapy added. The most frequent reasons for discontinuation were intolerance/side-effects, lack of efficacy, and therapeutic strategy. Combination therapy, usually hydroxycarbamide + anagrelide, is used in approximately 10% of all high-risk patients with essential thrombocythemia and may be a useful approach in treating patients for whom monotherapy is unsatisfactory. (Clinicaltrials.gov identifier:NCT00567502).
Figures
References
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haemopoietic and Lymphoid Tissues. 4th ed. IARC Press; 2008
-
- Harrison C. Rethinking disease definitions and therapeutic strategies in essential thrombocythemia and polycythemia vera. Hematology Am Soc Hematol Educ Program. 2010;2010:129–34 - PubMed
-
- Tefferi A, Vainchenker W. Myeloproliferative neoplasms: molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol. 2011;29(5):573–82 - PubMed
-
- Barbui T, Barosi G, Grossi A, Gugliotta L, Liberato LN, Marchetti M, et al. Practice guidelines for the therapy of essential thrombocythemia. A statement from the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica. 2004;89(2):215–32 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical