Eye-specific IOP-induced displacements and deformations of human lamina cribrosa
- PMID: 24334450
- PMCID: PMC3880011
- DOI: 10.1167/iovs.13-12724
Eye-specific IOP-induced displacements and deformations of human lamina cribrosa
Abstract
Purpose: To measure high-resolution eye-specific displacements and deformations induced within the human LC microstructure by an acute increase in IOP.
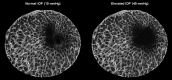
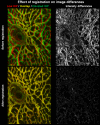
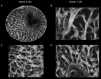
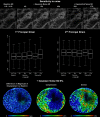
Methods: Six eyes from donors aged 23 to 82 were scanned using second harmonic-generated (SHG) imaging at various levels of IOP from 10 to 50 mm Hg. An image registration technique was developed, tested, and used to find the deformation mapping between maximum intensity projection images acquired at low and elevated IOP. The mappings were analyzed to determine the magnitude and distribution of the IOP-induced displacements and deformations and contralateral similarity.
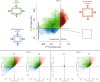
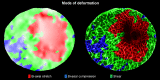
Results: Images of the LC were obtained and the registration technique was successful. IOP increases produced substantial, and potentially biologically significant, levels of in-plane LC stretch and compression (reaching 10%-25% medians and 20%-30% 75th percentiles). Deformations were sometimes highly focal and concentrated in regions as small as a few pores. Regions of largest displacement, stretch, compression, and shear did not colocalize. Displacements and strains were not normally distributed. Contralateral eyes did not always have more similar responses to IOP than unrelated eyes. Under elevated IOP, some LC regions were under bi-axial stretch, others under bi-axial compression.
Conclusions: We obtained eye-specific measurements of the complex effects of IOP on the LC with unprecedented resolution in uncut and unfixed human eyes. Our technique was robust to electronic and speckle noise. Elevated IOP produced substantial in-plane LC stretch and compression. Further research will explore the effects of IOP on the LC in a three-dimensional framework.
Keywords: biomechanics; glaucoma; lamina cribrosa; optic nerve head; second harmonic imaging; strain.
Figures





Comment in
-
A significant advance in the biomechanical evaluation of the optic nerve head.Invest Ophthalmol Vis Sci. 2014 Jan 7;55(1):215. doi: 10.1167/iovs.13-13789. Invest Ophthalmol Vis Sci. 2014. PMID: 24398251 No abstract available.
Similar articles
-
Cerebrospinal Fluid Pressure: Revisiting Factors Influencing Optic Nerve Head Biomechanics.Invest Ophthalmol Vis Sci. 2018 Jan 1;59(1):154-165. doi: 10.1167/iovs.17-22488. Invest Ophthalmol Vis Sci. 2018. PMID: 29332130 Free PMC article.
-
Modeling individual-specific human optic nerve head biomechanics. Part I: IOP-induced deformations and influence of geometry.Biomech Model Mechanobiol. 2009 Apr;8(2):85-98. doi: 10.1007/s10237-008-0120-7. Epub 2008 Feb 29. Biomech Model Mechanobiol. 2009. PMID: 18309526
-
IOP-induced regional displacements in the optic nerve head and correlation with peripapillary sclera thickness.Exp Eye Res. 2020 Nov;200:108202. doi: 10.1016/j.exer.2020.108202. Epub 2020 Aug 27. Exp Eye Res. 2020. PMID: 32861767 Free PMC article.
-
Biomechanics of the optic nerve head.Exp Eye Res. 2009 Apr;88(4):799-807. doi: 10.1016/j.exer.2009.02.003. Epub 2009 Feb 14. Exp Eye Res. 2009. PMID: 19217902 Review.
-
[Biomechanics of the lamina cribrosa: A determining factor in glaucomatous neuropathy. A review of the literature].J Fr Ophtalmol. 2023 Nov;46(9):1087-1098. doi: 10.1016/j.jfo.2023.05.026. Epub 2023 Oct 10. J Fr Ophtalmol. 2023. PMID: 37827943 Review. French.
Cited by
-
TRPV4 and TRPC1 channels mediate the response to tensile strain in mouse Müller cells.Cell Calcium. 2022 Jun;104:102588. doi: 10.1016/j.ceca.2022.102588. Epub 2022 Apr 5. Cell Calcium. 2022. PMID: 35398674 Free PMC article.
-
Factors associated with lamina cribrosa displacement after trabeculectomy.Graefes Arch Clin Exp Ophthalmol. 2019 Feb;257(2):435-436. doi: 10.1007/s00417-018-4199-y. Epub 2018 Nov 26. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 30478474 No abstract available.
-
Microstructural Deformations Within the Depth of the Lamina Cribrosa in Response to Acute In Vivo Intraocular Pressure Modulation.Invest Ophthalmol Vis Sci. 2022 May 2;63(5):25. doi: 10.1167/iovs.63.5.25. Invest Ophthalmol Vis Sci. 2022. PMID: 35604666 Free PMC article.
-
Gaze-Evoked Deformations in Optic Nerve Head Drusen: Repetitive Shearing as a Potential Factor in the Visual and Vascular Complications.Ophthalmology. 2018 Jun;125(6):929-937. doi: 10.1016/j.ophtha.2017.12.006. Epub 2018 Apr 2. Ophthalmology. 2018. PMID: 29361354 Free PMC article.
-
Lamina cribrosa in glaucoma.Curr Opin Ophthalmol. 2017 Mar;28(2):113-119. doi: 10.1097/ICU.0000000000000354. Curr Opin Ophthalmol. 2017. PMID: 27898470 Free PMC article. Review.
References
-
- Quigley HA. Glaucoma: macrocosm to microcosm the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2005; 46: 2662–2670 - PubMed
-
- Minckler DS, Bunt AH, Johanson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci. 1977; 16: 426–441 - PubMed
-
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999; 18: 39–57 - PubMed
-
- AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130: 429 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

