An efficient method for differentiation of human induced pluripotent stem cells into hepatocyte-like cells retaining drug metabolizing activity
- PMID: 24334537
- PMCID: PMC6594155
- DOI: 10.2133/dmpk.dmpk-13-rg-104
An efficient method for differentiation of human induced pluripotent stem cells into hepatocyte-like cells retaining drug metabolizing activity
Abstract
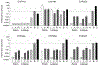
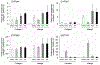
The use of human induced pluripotent stem (iPS) cells would be of great value for a variety of applications involving drug development studies. Several reports have been published on the differentiation of human iPS cells into hepatocyte-like cells; however, the cells were insufficient for application in drug metabolism studies. In this study, we aimed to establish effective methods for differentiation of human iPS cells into hepatocytes. Two human iPS cell lines were differentiated by addition of activin A, dimethyl sulfoxide, hepatocyte growth factor, oncostatin M, and dexamethasone. The differentiated cells expressed hepatocyte markers and drug-metabolizing enzymes, revealing that the human iPS cells were differentiated into hepatocyte-like cells. Expression of CYP3A4 and UGT1A1 mRNAs increased with treatment with typical inducers of the enzymes, and the response of the cells against the inducers was similar to that of human hepatocytes. Furthermore, the drug-metabolizing activity of CYP3A4, as monitored by testosterone 6β-hydroxylase activity, was elevated by these inducers. In conclusion, we established methods for differentiation of hepatocyte-like cells expressing drug metabolizing activity from human iPS cells. The hepatocyte-like cells derived from human iPS cells will be useful for drug metabolism studies.
Figures
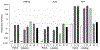

Similar articles
-
Histone deacetylase inhibitor valproic acid promotes the differentiation of human induced pluripotent stem cells into hepatocyte-like cells.PLoS One. 2014 Aug 1;9(8):e104010. doi: 10.1371/journal.pone.0104010. eCollection 2014. PLoS One. 2014. PMID: 25084468 Free PMC article.
-
Evaluation of human embryonic stem cell-derived hepatocyte-like cells for detection of CYP1A inducers.Drug Metab Pharmacokinet. 2012;27(6):598-604. doi: 10.2133/dmpk.dmpk-12-rg-017. Epub 2012 May 29. Drug Metab Pharmacokinet. 2012. PMID: 22673034
-
Enrichment of high-functioning human iPS cell-derived hepatocyte-like cells for pharmaceutical research.Biomaterials. 2018 Apr;161:24-32. doi: 10.1016/j.biomaterials.2018.01.019. Epub 2018 Jan 18. Biomaterials. 2018. PMID: 29421559
-
Pluripotent stem cell-derived hepatocyte-like cells.Biotechnol Adv. 2014 Mar-Apr;32(2):504-13. doi: 10.1016/j.biotechadv.2014.01.003. Epub 2014 Jan 16. Biotechnol Adv. 2014. PMID: 24440487 Free PMC article. Review.
-
[Generation of Zone-specific Hepatocyte-like Cells from Human Induced Pluripotent Stem Cells for Accurate Prediction of Drug-induced Hepatotoxicity].Yakugaku Zasshi. 2019;139(12):1509-1512. doi: 10.1248/yakushi.19-00170. Yakugaku Zasshi. 2019. PMID: 31787637 Review. Japanese.
Cited by
-
Human Placenta-Derived ECM Supports Tri-Lineage Differentiation of Human Induced Pluripotent Stem Cells.Int J Stem Cells. 2020 Nov 30;13(3):432-438. doi: 10.15283/ijsc20074. Int J Stem Cells. 2020. PMID: 32840229 Free PMC article.
-
An Efficient Method for the Differentiation of Human iPSC-Derived Endoderm toward Enterocytes and Hepatocytes.Cells. 2021 Apr 6;10(4):812. doi: 10.3390/cells10040812. Cells. 2021. PMID: 33917333 Free PMC article.
-
Consideration of Commercially Available Hepatocytes as Cell Sources for Liver-Microphysiological Systems by Comparing Liver Characteristics.Pharmaceutics. 2022 Dec 24;15(1):55. doi: 10.3390/pharmaceutics15010055. Pharmaceutics. 2022. PMID: 36678684 Free PMC article.
-
Induced pluripotent stem cells as an innovative model to study drug induced pancreatitis.World J Gastroenterol. 2021 Sep 21;27(35):5796-5802. doi: 10.3748/wjg.v27.i35.5796. World J Gastroenterol. 2021. PMID: 34629803 Free PMC article.
-
Hepatic differentiation of human iPSCs in different 3D models: A comparative study.Int J Mol Med. 2017 Dec;40(6):1759-1771. doi: 10.3892/ijmm.2017.3190. Epub 2017 Oct 16. Int J Mol Med. 2017. PMID: 29039463 Free PMC article.
References
-
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW and Daley GQ: Reprogramming of human somatic cells to pluripotency with defined factors. Nature, 451: 141–146 (2008). - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131: 861–872 (2007). - PubMed
-
- Takahashi K and Yamanaka S: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126: 663–676 (2006). - PubMed
-
- Ochiya T, Yamamoto Y and Banas A: Commitment of stem cells into functional hepatocytes. Differentiation, 79: 65–73 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
