Deletion of PDZD7 disrupts the Usher syndrome type 2 protein complex in cochlear hair cells and causes hearing loss in mice
- PMID: 24334608
- PMCID: PMC3976334
- DOI: 10.1093/hmg/ddt629
Deletion of PDZD7 disrupts the Usher syndrome type 2 protein complex in cochlear hair cells and causes hearing loss in mice
Abstract
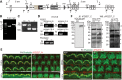
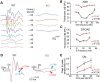
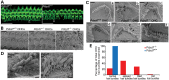
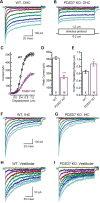
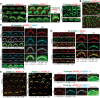
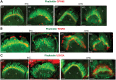
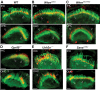
Usher syndrome type 2 (USH2) is the predominant form of USH, a leading genetic cause of combined deafness and blindness. PDZD7, a paralog of two USH causative genes, USH1C and USH2D (WHRN), was recently reported to be implicated in USH2 and non-syndromic deafness. It encodes a protein with multiple PDZ domains. To understand the biological function of PDZD7 and the pathogenic mechanism caused by PDZD7 mutations, we generated and thoroughly characterized a Pdzd7 knockout mouse model. The Pdzd7 knockout mice exhibit congenital profound deafness, as assessed by auditory brainstem response, distortion product otoacoustic emission and cochlear microphonics tests, and normal vestibular function, as assessed by their behaviors. Lack of PDZD7 leads to the disorganization of stereocilia bundles and a reduction in mechanotransduction currents and sensitivity in cochlear outer hair cells. At the molecular level, PDZD7 determines the localization of the USH2 protein complex, composed of USH2A, GPR98 and WHRN, to ankle links in developing cochlear hair cells, likely through its direct interactions with these three proteins. The localization of PDZD7 to the ankle links of cochlear hair bundles also relies on USH2 proteins. In photoreceptors of Pdzd7 knockout mice, the three USH2 proteins largely remain unchanged at the periciliary membrane complex. The electroretinogram responses of both rod and cone photoreceptors are normal in knockout mice at 1 month of age. Therefore, although the organization of the USH2 complex appears different in photoreceptors, it is clear that PDZD7 plays an essential role in organizing the USH2 complex at ankle links in developing cochlear hair cells. GenBank accession numbers: KF041446, KF041447, KF041448, KF041449, KF041450, KF041451.
Figures



References
-
- Boughman J.A., Vernon M., Shaver K.A. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J. Chronic Dis. 1983;36:595–603. - PubMed
-
- Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. - PubMed
-
- Keats B.J., Corey D.P. The usher syndromes. Am. J. Med. Genet. 1999;89:158–166. - PubMed
-
- Eudy J.D., Weston M.D., Yao S., Hoover D.M., Rehm H.L., Ma-Edmonds M., Yan D., Ahmad I., Cheng J.J., Ayuso C., et al. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science. 1998;280:1753–1757. - PubMed
-
- Reiners J., Nagel-Wolfrum K., Jurgens K., Marker T., Wolfrum U. Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp. Eye Res. 2006;83:97–119. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

