Age-associated stresses induce an anti-inflammatory senescent phenotype in endothelial cells
- PMID: 24334613
- PMCID: PMC3883707
- DOI: 10.18632/aging.100622
Age-associated stresses induce an anti-inflammatory senescent phenotype in endothelial cells
Abstract
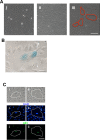
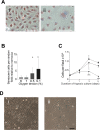
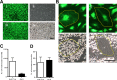
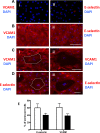
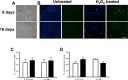
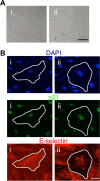
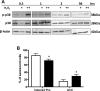
Age is the greatest risk factor for cardiovascular disease. In addition, inflammation and age (senescence) have been linked at both the clinical and molecular levels. In general, senescent cells have been described as pro-inflammatory based on their senescence associated secretory phenotype (SASP). However, we have previously shown that senescence induced by overexpression ofSENEX (or ARHGAP18), in endothelial cells results in an anti-inflammatory phenotype. We have investigated, at the individual cellular level, the senescent phenotype of endothelial cells following three of the chief signals associated with ageing; oxidative stress, disturbed flow and hypoxia. All three stimuli induce senescence and, based on neutrophil adhesion and expression of the adhesion molecules E-selectin and VCAM-1, a population of senescent cells is seen that is resistant to inflammatory stimuli and thus we define as anti-inflammatory. The proportion of anti-inflammatory cells increases with time but remains stable at approximately 50% by eight days after induction of senescence, suggesting that these are stable phenotypes of endothelial cell senescence. Similar to other senescent cell types, p38MAPK blockade inhibits the development of the pro-inflammatory phenotype but unique to EC, there is a corresponding increase in the number of anti-inflammatory senescent cells. Thus stress-induced senescent endothelial cells display a mosaic of inflammatory phenotypes. The anti-inflammatory population suggests that senescent endothelial cells may have an unique protective role, to inhibit uncontrolled proliferation and to limit the local inflammatory response.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures


Similar articles
-
Caveolae control the anti-inflammatory phenotype of senescent endothelial cells.Aging Cell. 2015 Feb;14(1):102-11. doi: 10.1111/acel.12270. Epub 2014 Nov 19. Aging Cell. 2015. PMID: 25407919 Free PMC article.
-
Senolytics and Senomorphics Targeting p38MAPK/NF-κB Pathway Protect Endothelial Cells from Oxidative Stress-Mediated Premature Senescence.Cells. 2024 Jul 31;13(15):1292. doi: 10.3390/cells13151292. Cells. 2024. PMID: 39120322 Free PMC article.
-
Stress-induced premature senescence mediated by a novel gene, SENEX, results in an anti-inflammatory phenotype in endothelial cells.Blood. 2010 Nov 11;116(19):4016-24. doi: 10.1182/blood-2009-11-252700. Epub 2010 Jul 27. Blood. 2010. PMID: 20664062
-
Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP).Cell Signal. 2012 Apr;24(4):835-45. doi: 10.1016/j.cellsig.2011.12.006. Epub 2011 Dec 11. Cell Signal. 2012. PMID: 22182507 Review.
-
Senescent endothelial cells: Potential modulators of immunosenescence and ageing.Ageing Res Rev. 2016 Aug;29:13-25. doi: 10.1016/j.arr.2016.05.011. Epub 2016 May 26. Ageing Res Rev. 2016. PMID: 27235855 Review.
Cited by
-
Role of p38 mitogen-activated protein kinase in vascular endothelial aging: interaction with Arginase-II and S6K1 signaling pathway.Aging (Albany NY). 2015 Jan;7(1):70-81. doi: 10.18632/aging.100722. Aging (Albany NY). 2015. PMID: 25635535 Free PMC article.
-
Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases.Int J Mol Sci. 2022 Dec 11;23(24):15722. doi: 10.3390/ijms232415722. Int J Mol Sci. 2022. PMID: 36555364 Free PMC article. Review.
-
The role of hypoxic signalling in metastasis: towards translating knowledge of basic biology into novel anti-tumour strategies.Clin Exp Metastasis. 2018 Oct;35(7):563-599. doi: 10.1007/s10585-018-9930-x. Epub 2018 Aug 31. Clin Exp Metastasis. 2018. PMID: 30171389 Review.
-
Caveolae control the anti-inflammatory phenotype of senescent endothelial cells.Aging Cell. 2015 Feb;14(1):102-11. doi: 10.1111/acel.12270. Epub 2014 Nov 19. Aging Cell. 2015. PMID: 25407919 Free PMC article.
-
Outcomes of Deferoxamine Action on H2O2-Induced Growth Inhibition and Senescence Progression of Human Endometrial Stem Cells.Int J Mol Sci. 2021 Jun 3;22(11):6035. doi: 10.3390/ijms22116035. Int J Mol Sci. 2021. PMID: 34204881 Free PMC article.
References
-
- Voghel G, Thorin-Trescases Trescases, Farhat N, Nguyen A, Villeneuve L, Mamarbachi AM, Fortier A, Perrault LP, Carrier M, Thorin E. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mechanisms of ageing and development. 2007;128:662–671. - PubMed
-
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. - PubMed
-
- Chen J, Brodsky SV, Goligorsky DM, Hampel DJ, Li H, Gross SS, Goligorsky MS. Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells. Circulation research. 2002;90:1290–1298. - PubMed
-
- Kuki S, Imanishi T, Kobayashi K, Matsuo Y, Obana M, Akasaka T. Hyperglycemia accelerated endothelial progenitor cell senescence via the activation of p38 mitogen-activated protein kinase. Circ J. 2006;70:1076–1081. - PubMed
-
- Scalera F, Martens-Lobenhoffer Lobenhoffer, Tager M, Bukowska A, Lendeckel U, Bode-Boger SM. Effect of L-arginine on asymmetric dimethylarginine (ADMA) or homocysteine-accelerated endothelial cell aging. Biochemical and biophysical research communications. 2006;345:1075–1082. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

