The Legionella pneumophila collagen-like protein mediates sedimentation, autoaggregation, and pathogen-phagocyte interactions
- PMID: 24334670
- PMCID: PMC3911070
- DOI: 10.1128/AEM.03254-13
The Legionella pneumophila collagen-like protein mediates sedimentation, autoaggregation, and pathogen-phagocyte interactions
Abstract
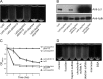
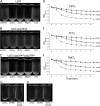
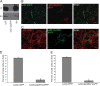
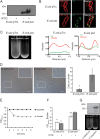
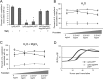
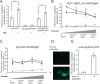
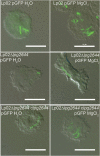
Although only partially understood, multicellular behavior is relatively common in bacterial pathogens. Bacterial aggregates can resist various host defenses and colonize their environment more efficiently than planktonic cells. For the waterborne pathogen Legionella pneumophila, little is known about the roles of autoaggregation or the parameters which allow cell-cell interactions to occur. Here, we determined the endogenous and exogenous factors sufficient to allow autoaggregation to take place in L. pneumophila. We show that isolates from Legionella species which do not produce the Legionella collagen-like protein (Lcl) are deficient in autoaggregation. Targeted deletion of the Lcl-encoding gene (lpg2644) and the addition of Lcl ligands impair the autoaggregation of L. pneumophila. In addition, Lcl-induced autoaggregation requires divalent cations. Escherichia coli producing surface-exposed Lcl is able to autoaggregate and shows increased biofilm production. We also demonstrate that L. pneumophila infection of Acanthamoeba castellanii and Hartmanella vermiformis is potentiated under conditions which promote Lcl dependent autoaggregation. Overall, this study shows that L. pneumophila is capable of autoaggregating in a process that is mediated by Lcl in a divalent-cation-dependent manner. It also reveals that Lcl potentiates the ability of L. pneumophila to come in contact, attach, and infect amoebae.
Figures

Similar articles
-
The autoinducer synthase LqsA and putative sensor kinase LqsS regulate phagocyte interactions, extracellular filaments and a genomic island of Legionella pneumophila.Environ Microbiol. 2010 May;12(5):1243-59. doi: 10.1111/j.1462-2920.2010.02167.x. Epub 2010 Feb 9. Environ Microbiol. 2010. PMID: 20148929
-
The Legionella pneumophila orphan sensor kinase LqsT regulates competence and pathogen-host interactions as a component of the LAI-1 circuit.Environ Microbiol. 2013 Feb;15(2):646-62. doi: 10.1111/j.1462-2920.2012.02889.x. Epub 2012 Oct 4. Environ Microbiol. 2013. PMID: 23033905
-
Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila.Cell Microbiol. 2006 Aug;8(8):1228-40. doi: 10.1111/j.1462-5822.2006.00703.x. Cell Microbiol. 2006. PMID: 16882028
-
Legionella pneumophila adaptation to intracellular life and the host response: clues from genomics and transcriptomics.FEBS Lett. 2007 Jun 19;581(15):2829-38. doi: 10.1016/j.febslet.2007.05.026. Epub 2007 May 21. FEBS Lett. 2007. PMID: 17531986 Review.
-
Acanthamoeba and Dictyostelium as Cellular Models for Legionella Infection.Front Cell Infect Microbiol. 2018 Mar 2;8:61. doi: 10.3389/fcimb.2018.00061. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29552544 Free PMC article. Review.
Cited by
-
Vermamoeba vermiformis: a Free-Living Amoeba of Interest.Microb Ecol. 2018 Nov;76(4):991-1001. doi: 10.1007/s00248-018-1199-8. Epub 2018 May 8. Microb Ecol. 2018. PMID: 29737382 Review.
-
Establishing the Median Infectious Dose and Characterizing the Clinical Manifestations of Mouse, Rat, Cow, and Human Corynebacterium bovis Isolates in Select Immunocompromised Mouse Strains.Comp Med. 2023 Jun 1;73(3):200-215. doi: 10.30802/AALAS-CM-22-000115. Epub 2023 Jun 1. Comp Med. 2023. PMID: 37277182 Free PMC article.
-
Environmental (Saprozoic) Pathogens of Engineered Water Systems: Understanding Their Ecology for Risk Assessment and Management.Pathogens. 2015 Jun 19;4(2):390-405. doi: 10.3390/pathogens4020390. Pathogens. 2015. PMID: 26102291 Free PMC article. Review.
-
Fungal biofilm morphology impacts hypoxia fitness and disease progression.Nat Microbiol. 2019 Dec;4(12):2430-2441. doi: 10.1038/s41564-019-0558-7. Epub 2019 Sep 23. Nat Microbiol. 2019. PMID: 31548684 Free PMC article.
-
Polymorphisms of a Collagen-Like Adhesin Contributes to Legionella pneumophila Adhesion, Biofilm Formation Capacity and Clinical Prevalence.Front Microbiol. 2019 Apr 5;10:604. doi: 10.3389/fmicb.2019.00604. eCollection 2019. Front Microbiol. 2019. PMID: 31024468 Free PMC article.
References
-
- Hélaine S, Carbonnelle E, Prouvensier L, Beretti J, Nassif X, Pelicic V. 2005. PilX, a pilus-associated protein essential for bacterial aggregation, is a key to pilus-facilitated attachment of Neisseria meningitidis to human cells. Mol. Microbiol. 55:65–77. 10.1111/j.1365-2958.2004.04372.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

