Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging
- PMID: 24335072
- PMCID: PMC3864379
- DOI: 10.1167/iovs.13-12757
Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging
Abstract
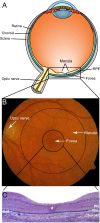
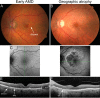
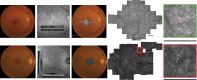
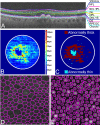
Age-related macular degeneration is the leading cause of irreversible visual dysfunction in individuals over 65 in Western Society. Patients with AMD are classified as having early stage disease (early AMD), in which visual function is affected, or late AMD (generally characterized as either "wet" neovascular AMD, "dry" atrophic AMD or both), in which central vision is severely compromised or lost. Until recently, there have been no therapies available to treat the disorder(s). Now, the most common wet form of late-stage AMD, choroidal neovascularization, generally responds to treatment with anti-vascular endothelial growth factor therapies. Nevertheless, there are no current therapies to restore lost vision in eyes with advanced atrophic AMD. Oral supplementation with the Age-Related Eye Disease Study (AREDS) or AREDS2 formulation (antioxidant vitamins C and E, lutein, zeaxanthin, and zinc) has been shown to reduce the risk of progression to advanced AMD, although the impact was in neovascular rather than atrophic AMD. Recent findings, however, have demonstrated several features of early AMD that are likely to be druggable targets for treatment. Studies have established that much of the genetic risk for AMD is associated with complement genes. Consequently, several complement-based therapeutic treatment approaches are being pursued. Potential treatment strategies against AMD deposit formation and protein and/or lipid deposition will be discussed, including anti-amyloid therapies. In addition, the role of autophagy in AMD and prevention of oxidative stress through modulation of the antioxidant system will be explored. Finally, the success of these new therapies in clinical trials and beyond relies on early detection, disease typing, and predicting disease progression, areas that are currently being rapidly transformed by improving imaging modalities and functional assays.
Keywords: autophagy; complement; drusen; functional imaging; therapeutic targets.
Figures
References
-
- Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997; 104: 7–21 - PubMed
-
- Klein R, Klein BE, Franke T. The relationship of cardiovascular disease and its risk factors to age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1993; 100: 406–414 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

