Effects of diabetes on the eye
- PMID: 24335073
- PMCID: PMC3864380
- DOI: 10.1167/iovs.13-12979
Effects of diabetes on the eye
Abstract
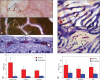
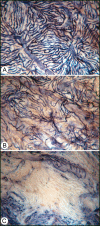
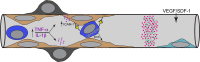
Hyperglycemia has toxic effects on almost all cells in the body. Ophthalmic complications of hyperglycemia are most profound in cornea and retina. Seventy percent of diabetics suffer from corneal complications, collectively called diabetic keratopathy, which includes include recurrent erosions, delayed wound healing, ulcers, and edema. Confocal microscopy has permitted in vivo imaging of corneal nerves, which are also affected in diabetic subjects. Gene therapies upregulating MNNG HOS transforming gene (cMet) and/or downregulating MMP10 and cathepsin S are potential future therapies for diabetic keratopathy. Diabetic retinopathy (DR) is the most common cause of blindness in people over the age of 50. There is accumulating evidence that DR is an inflammatory disease. The initial events in animal models of DR are increased vascular permeability and leukostasis. This binding of leukocytes to the endothelium results from an increase in intracellular adhesion molecule-1 (ICAM-1) on the retinal capillary endothelium (EC) and expression of CD11/CD18 on the surface of the activated leukocyte. We have observed polymorphonuclear leukocytes (PMNs) at sites of EC vascular dysfunction in diabetic retinas as well as choroid. Anti-inflammatory drugs like etanercept, aspirin, or meloxicam reduce leukostasis and EC death. Future therapies may include repopulation of the acellular capillaries after EC and pericyte death with vascular progenitors made from the patient's own blood cells.
Keywords: choroidopathy; cornea; diabetes; inflammation; retinopathy.
Figures

References
-
- Midena E, Brugin E, Ghirlando A, Sommavilla M, Avogaro A. Corneal diabetic neuropathy: a confocal microscopy study. J Refract Surg. 2006; 22: S1047–S1052 - PubMed
-
- Midena E, Cortez M, Miotto S, Gambato C, Cavarzeran F, Ghirlando A. Confocal microscopy of corneal sub-basal nerve plexus: a quantitative and qualitative analysis in healthy and pathologic eyes. J Refract Surg. 2009; 25: S125–S130 - PubMed
-
- Chikama T, Wakuta M, Liu Y, Nishida T. Deviated mechanism of wound healing in diabetic corneas. Cornea. 2007; 26: S75–S81 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

