Tumor microenvironment-associated modifications of alternative splicing
- PMID: 24335142
- PMCID: PMC3895271
- DOI: 10.1261/rna.042168.113
Tumor microenvironment-associated modifications of alternative splicing
Abstract
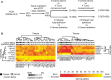
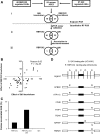
Pre-mRNA alternative splicing is modified in cancer, but the origin and specificity of these changes remain unclear. Here, we probed ovarian tumors to identify cancer-associated splicing isoforms and define the mechanism by which splicing is modified in cancer cells. Using high-throughput quantitative PCR, we monitored the expression of splice variants in laser-dissected tissues from ovarian tumors. Surprisingly, changes in alternative splicing were not limited to the tumor tissues but were also found in the tumor microenvironment. Changes in the tumor-associated splicing events were found to be regulated by splicing factors that are differentially expressed in cancer tissues. Overall, ∼20% of the alternative splicing events affected by the down-regulation of the splicing factors QKI and RBFOX2 were altered in the microenvironment of ovarian tumors. Together, our results indicate that the tumor microenvironment undergoes specific changes in alternative splicing orchestrated by a limited number of splicing factors.
Keywords: RNA binding proteins; alternative splicing; laser capture microdissection; ovarian cancer; tumor microenvironment.
Figures




References
-
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC 2001. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr Rev 22: 255–288 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
