Low-frequency stimulation induces long-term depression and slow onset long-term potentiation at perforant path-dentate gyrus synapses in vivo
- PMID: 24335215
- PMCID: PMC3949311
- DOI: 10.1152/jn.00941.2012
Low-frequency stimulation induces long-term depression and slow onset long-term potentiation at perforant path-dentate gyrus synapses in vivo
Abstract
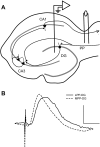
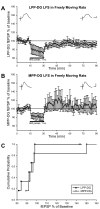
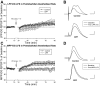
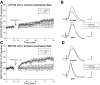
The expression of homosynaptic long-term depression (LTD) is thought to mediate a crucial role in sustaining memory function. Our in vivo investigations of LTD expression at lateral (LPP) and medial perforant path (MPP) synapses in the dentate gyrus (DG) corroborate prior demonstrations that PP-DG LTD is difficult to induce in intact animals. In freely moving animals, LTD expression occurred inconsistently among LPP-DG and MPP-DG responses. Interestingly, following acute electrode implantation in anesthetized rats, low-frequency stimulation (LFS; 900 pulses, 1 Hz) promotes slow-onset LTP at both MPP-DG and LPP-DG synapses that utilize distinct induction mechanisms. Systemic administration of the N-methyl-d-aspartate (NMDA) receptor antagonist (+/-)-cyclopiperidine-6-piperiperenzine (CPP; 10 mg/kg) 90 min before LFS selectively blocked MPP-DG but not LPP-DG slow onset LTP, suggesting MPP-DG synapses express a NMDA receptor-dependent slow onset LTP whereas LPP-DG slow onset LTP induction is NMDA receptor independent. In experiments where paired-pulse LFS (900 paired pulses, 200-ms paired-pulse interval) was used to induce LTD, paired-pulse LFS of the LPP resulted in rapid onset LTP of DG responses, whereas paired-pulse LFS of the MPP induced slow onset LTP of DG responses. Although LTD observations were very rare following acute electrode implantation in anesthetized rats, LPP-DG LTD was demonstrated in some anesthetized rats with previously implanted electrodes. Together, our data indicate in vivo PP-DG LTD expression is an inconsistent phenomenon that is primarily observed in recovered animals, suggesting perturbation of the dentate through surgery-related tissue trauma influences both LTD incidence and LTP induction at PP-DG synapses in vivo.
Keywords: LTD; dentate gyrus; in vivo; perforant path; slow onset LTP.
Figures



Similar articles
-
Preferential frequency-dependent induction of synaptic depression by the lateral perforant path and of synaptic potentiation by the medial perforant path inputs to the dentate gyrus.Hippocampus. 2021 Sep;31(9):957-981. doi: 10.1002/hipo.23338. Epub 2021 May 18. Hippocampus. 2021. PMID: 34002905
-
Long-term potentiation in direct perforant path projections to the hippocampal CA3 region in vivo.J Neurophysiol. 2002 Feb;87(2):669-78. doi: 10.1152/jn.00938.2000. J Neurophysiol. 2002. PMID: 11826036
-
Fimbrial control of bidirectional synaptic plasticity of medial perforant path-dentate transmission.Synapse. 2003 Mar;47(3):163-8. doi: 10.1002/syn.10168. Synapse. 2003. PMID: 12494398
-
Comparison of cellular mechanisms of long-term depression of synaptic strength at perforant path-granule cell and Schaffer collateral-CA1 synapses.Prog Brain Res. 2007;163:473-500. doi: 10.1016/S0079-6123(07)63026-X. Prog Brain Res. 2007. PMID: 17765734 Review.
-
Astrocyte control of the entorhinal cortex-dentate gyrus circuit: Relevance to cognitive processing and impairment in pathology.Glia. 2022 Aug;70(8):1536-1553. doi: 10.1002/glia.24128. Epub 2021 Dec 14. Glia. 2022. PMID: 34904753 Free PMC article. Review.
Cited by
-
The Impact of Synaptic Zn2+ Dynamics on Cognition and Its Decline.Int J Mol Sci. 2017 Nov 14;18(11):2411. doi: 10.3390/ijms18112411. Int J Mol Sci. 2017. PMID: 29135924 Free PMC article. Review.
-
Systems modeling predicts that mitochondria ER contact sites regulate the postsynaptic energy landscape.NPJ Syst Biol Appl. 2021 Jun 2;7(1):26. doi: 10.1038/s41540-021-00185-7. NPJ Syst Biol Appl. 2021. PMID: 34078916 Free PMC article.
-
A review of combined neuromodulation and physical therapy interventions for enhanced neurorehabilitation.Front Hum Neurosci. 2023 Jul 21;17:1151218. doi: 10.3389/fnhum.2023.1151218. eCollection 2023. Front Hum Neurosci. 2023. PMID: 37545593 Free PMC article. Review.
-
Lentiviral Expression of Rabies Virus Glycoprotein in the Rat Hippocampus Strengthens Synaptic Plasticity.Cell Mol Neurobiol. 2022 Jul;42(5):1429-1440. doi: 10.1007/s10571-020-01032-9. Epub 2021 Jan 19. Cell Mol Neurobiol. 2022. PMID: 33462779 Free PMC article.
-
Synaptic Plasticity at Inhibitory Synapses in the Ventral Tegmental Area Depends upon Stimulation Site.eNeuro. 2019 Nov 15;6(6):ENEURO.0137-19.2019. doi: 10.1523/ENEURO.0137-19.2019. Print 2019 Nov/Dec. eNeuro. 2019. PMID: 31619451 Free PMC article.
References
-
- Abraham WC, Goddard GV. Asymmetric relationships between homosynaptic long-term potentiation and heterosynaptic long-term depression. Nature 305: 717–719, 1983 - PubMed
-
- Abraham WC, McNaughton N. Differences in synaptic transmission between medial and lateral components of the perforant path. Brain Res 303: 251–260, 1984 - PubMed
-
- Abraham WC. Induction of heterosynaptic and homosynaptic long-term depression in hippocampal subregions in vivo. J Physiol (Paris) 90: 305–306, 1996 - PubMed
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126–130, 1996 - PubMed
-
- Abraham WC, Mason-Parker SE, Logan B. Low-frequency stimulation does not readily cause long-term depression or depotentiation in the dentate gyrus of awake rats. Brain Res 722: 217–221, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

