Reorganization of hippocampal functional connectivity with transition to chronic back pain
- PMID: 24335219
- PMCID: PMC3949236
- DOI: 10.1152/jn.00611.2013
Reorganization of hippocampal functional connectivity with transition to chronic back pain
Abstract
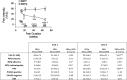
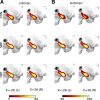
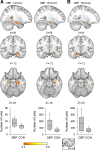
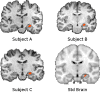
The hippocampus has been shown to undergo significant changes in rodent models of neuropathic pain; however, the role of the hippocampus in human chronic pain and its contribution to pain chronification have remained unexplored. Here we examine hippocampal processing during a simple visual attention task. We used functional MRI to identify intrinsic and extrinsic hippocampal functional connectivity (synchronous neural activity), comparing subacute back pain (SBP, back pain 1-4 mo) and chronic back pain (CBP, back pain >10 yr) patients to control (CON) subjects. Both groups showed more extensive hippocampal connectivity than CON subjects. We then examined the evolution of hippocampal connectivity longitudinally in SBP patients who recovered (SBPr, back pain decreased >20% in 1 yr) and those with persistent pain (SBPp). We found that SBPp and SBPr subjects have distinct changes in hippocampal-cortical connectivity over 1 yr; specifically, SBPp subjects showed large decreases in hippocampal connectivity with medial prefrontal cortex (HG-mPFC). Furthermore, in SBP patients the strength of HG-mPFC reflected variations in back pain over the year. These relationships were replicated when examined in a different task performed by SBP patients (rating fluctuations of back pain), indicating that functional connectivity of the hippocampus changes robustly in subacute pain and the nature of these changes depends on whether or not patients recover from SBP. The observed reorganization of processing within the hippocampus and between the hippocampus and the cortex seems to contribute to the transition from subacute to chronic pain and may also underlie learning and emotional abnormalities associated with chronic pain.
Keywords: back pain; functional connectivity; hippocampus; medial prefrontal cortex; transition to chronic pain.
Figures
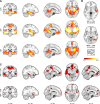
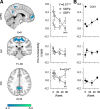

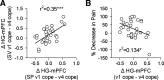
References
-
- Abrahams S, Morris RG, Polkey CE, Jarosz JM, Cox TC, Graves M, Pickering A. Hippocampal involvement in spatial and working memory: a structural MRI analysis of patients with unilateral mesial temporal lobe sclerosis. Brain Cogn 41: 39–65, 1999 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

