STAT1 interaction with E3-14.7K in monocytes affects the efficacy of oncolytic adenovirus
- PMID: 24335311
- PMCID: PMC3911530
- DOI: 10.1128/JVI.02829-13
STAT1 interaction with E3-14.7K in monocytes affects the efficacy of oncolytic adenovirus
Erratum in
-
Correction for Spurrell et al., "STAT1 Interaction with E3-14.7K in Monocytes Affects the Efficacy of Oncolytic Adenovirus".J Virol. 2017 Sep 27;91(20):e00670-17. doi: 10.1128/JVI.00670-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28956778 Free PMC article. No abstract available.
Abstract
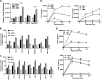
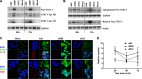
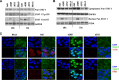
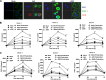
Oncolytic viruses based on adenovirus type 5 (Ad5) have been developed as a new class of therapeutic agents for cancers that are resistant to conventional therapies. Clinical experience shows that these agents are safe, but virotherapy alone has not achieved long-term cure in cancer patients. The vast majority of oncolytic adenoviruses used in clinical trials to date have deletion of the E3B genes. It has been demonstrated that the antitumor potency of the E3B-deleted mutant (dl309) is inferior to adenovirus with E3B genes intact. Tumors treated with dl309 show markedly greater macrophage infiltration than E3B-intact adenovirus. However, the functional mechanisms for this were not previously known. Here, we demonstrate that deletion of E3B genes increases production of chemokines by monocytes after adenovirus infection and increases monocyte migration. The E3B 14,700-Da protein (E3B-14.7K) inhibits STAT1 function by preventing its phosphorylation and nuclear translocation. The STAT1 inhibitor, fludarabine, rescues the effect of E3B-14.7K deletion by downregulating target chemokine expression in human and murine monocytes and results in an enhanced antitumor efficacy with dl309 in vivo. These findings have important implications for clinical use of E3B-deleted oncolytic adenovirus and other E3B-deleted adenovirus vector-based therapy.
Figures


References
-
- Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN, Chiocca EA. 2000. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J. Virol. 74:4765–4775. 10.1128/JVI.74.10.4765-4775.2000 - DOI - PMC - PubMed
-
- Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, Harsh GR, IV, Louis DN, Bartus RT, Hochberg FH, Chiocca EA. 1999. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat. Med. 5:881–887. 10.1038/11320 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

