FTY720 (s)-phosphonate preserves sphingosine 1-phosphate receptor 1 expression and exhibits superior barrier protection to FTY720 in acute lung injury
- PMID: 24335440
- PMCID: PMC4144721
- DOI: 10.1097/CCM.0000000000000097
FTY720 (s)-phosphonate preserves sphingosine 1-phosphate receptor 1 expression and exhibits superior barrier protection to FTY720 in acute lung injury
Abstract
Objective: Effective therapies are needed to reverse the increased vascular permeability that characterizes acute inflammatory diseases such as acute lung injury. FTY720 is a pharmaceutical analog of the potent barrier-enhancing phospholipid, sphingosine 1-phosphate. Because both FTY720 and sphingosine 1-phosphate have properties that may limit their usefulness in patients with acute lung injury, alternative compounds are needed for therapeutic use. The objective of this study is to characterize the effects of FTY720 (S)-phosphonate, a novel analog of FTY720-phosphate, on variables of pulmonary vascular permeability in vitro and alveolar-capillary permeability in vivo.
Setting: University-affiliated research institute.
Subjects: Cultured human pulmonary endothelial cells; C57BL/6 mice.
Interventions: Endothelial cells were stimulated with sphingosine 1-phosphate receptor 1 agonists to determine effects on sphingosine 1-phosphate receptor 1 expression. Acute lung injury was induced in C57BL/6 mice with bleomycin to assess effects of sphingosine 1-phosphate receptor 1 agonists.
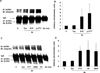
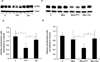
Measurements and main results: FTY720 (S)-phosphonate potently increases human pulmonary endothelial cell barrier function in vitro as measured by transendothelial electrical resistance. Reduction of sphingosine 1-phosphate receptor 1 with small interference RNA significantly attenuates this transendothelial electrical resistance elevation. FTY720 (S)-phosphonate maintains endothelial sphingosine 1-phosphate receptor 1 protein expression in contrast to greater than 50% reduction after incubation with sphingosine 1-phosphate, FTY720, or other sphingosine 1-phosphate receptor 1 agonists. FTY720 (S)-phosphonate does not induce β-arrestin recruitment, sphingosine 1-phosphate receptor 1 ubiquitination, and proteosomal degradation that occur after other agonists. Intraperitoneal administration of FTY720 (S)-phosphonate every other day for 1 week in normal or bleomycin-injured mice maintains significantly higher lung sphingosine 1-phosphate receptor 1 expression compared with FTY720. FTY720 fails to protect against bleomycin-induced acute lung injury in mice, while FTY720 (S)-phosphonate significantly decreases lung leak and inflammation.
Conclusion: FTY720 (S)-phosphonate is a promising barrier-promoting agent that effectively maintains sphingosine 1-phosphate receptor 1 levels and improves outcomes in the bleomycin model of acute lung injury.
Figures






Similar articles
-
Junctional complex and focal adhesion rearrangement mediates pulmonary endothelial barrier enhancement by FTY720 S-phosphonate.Microvasc Res. 2015 May;99:102-9. doi: 10.1016/j.mvr.2015.03.007. Epub 2015 Apr 7. Microvasc Res. 2015. PMID: 25862132 Free PMC article.
-
Synthetic analogs of FTY720 [2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol] differentially regulate pulmonary vascular permeability in vivo and in vitro.J Pharmacol Exp Ther. 2009 Oct;331(1):54-64. doi: 10.1124/jpet.109.153544. Epub 2009 Jul 10. J Pharmacol Exp Ther. 2009. PMID: 19592667 Free PMC article.
-
Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor.Cell Signal. 2007 Aug;19(8):1754-64. doi: 10.1016/j.cellsig.2007.03.011. Epub 2007 Apr 6. Cell Signal. 2007. PMID: 17475445 Free PMC article.
-
Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury.Am J Respir Cell Mol Biol. 2013 Jul;49(1):6-17. doi: 10.1165/rcmb.2012-0411TR. Am J Respir Cell Mol Biol. 2013. PMID: 23449739 Free PMC article. Review.
-
FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function.Am J Transplant. 2004 Jul;4(7):1019-25. doi: 10.1111/j.1600-6143.2004.00476.x. Am J Transplant. 2004. PMID: 15196057 Review.
Cited by
-
Fingolimod does not prevent syndecan-4 shedding from the endothelial glycocalyx in a cultured human umbilical vein endothelial cell model of vascular injury.Intensive Care Med Exp. 2022 Aug 18;10(1):34. doi: 10.1186/s40635-022-00462-7. Intensive Care Med Exp. 2022. PMID: 35980492 Free PMC article.
-
Sphingosine-1-phosphate receptor-independent lung endothelial cell barrier disruption induced by FTY720 regioisomers.Pulm Circ. 2020 Feb 10;10(1):10.1177_2045894020905521. doi: 10.1177/2045894020905521. eCollection 2020 Jan-Mar. Pulm Circ. 2020. PMID: 32095229 Free PMC article.
-
Sphingosine-1-Phosphate Treatment Can Ameliorate Microvascular Leakage Caused by Combined Alcohol Intoxication and Hemorrhagic Shock.Sci Rep. 2017 Jun 22;7(1):4078. doi: 10.1038/s41598-017-04157-y. Sci Rep. 2017. PMID: 28642485 Free PMC article.
-
Junctional complex and focal adhesion rearrangement mediates pulmonary endothelial barrier enhancement by FTY720 S-phosphonate.Microvasc Res. 2015 May;99:102-9. doi: 10.1016/j.mvr.2015.03.007. Epub 2015 Apr 7. Microvasc Res. 2015. PMID: 25862132 Free PMC article.
-
Pulmonary endothelial cell barrier enhancement by novel FTY720 analogs: methoxy-FTY720, fluoro-FTY720, and β-glucuronide-FTY720.Chem Phys Lipids. 2015 Oct;191:16-24. doi: 10.1016/j.chemphyslip.2015.08.004. Epub 2015 Aug 10. Chem Phys Lipids. 2015. PMID: 26272033 Free PMC article.
References
-
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005;353(16):1685–1693. - PubMed
-
- Gimbrone MA, Jr., Aster RH, Cotran RS, et al. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature. 1969;222(5188):33–36. - PubMed
-
- Lo SK, Burhop KE, Kaplan JE, et al. Role of platelets in maintenance of pulmonary vascular permeability to protein. Am J Physiol. 1988;254(4 Pt 2):H763–H771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

