Rapid kill of malaria parasites by artemisinin and semi-synthetic endoperoxides involves ROS-dependent depolarization of the membrane potential
- PMID: 24335485
- PMCID: PMC3956377
- DOI: 10.1093/jac/dkt486
Rapid kill of malaria parasites by artemisinin and semi-synthetic endoperoxides involves ROS-dependent depolarization of the membrane potential
Abstract
Objectives: Artemisinin and artemisinin semi-synthetic derivatives (collectively known as endoperoxides) are first-line antimalarials for the treatment of uncomplicated and severe malaria. Endoperoxides display very fast killing rates and are generally recalcitrant to parasite resistance development. These key pharmacodynamic features are a result of a complex mechanism of action, the details of which lack consensus. Here, we report on the primary physiological events leading to parasite death.
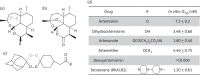
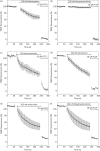
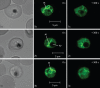
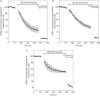
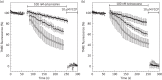
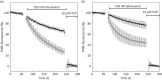
Methods: Parasite mitochondrial (ΔΨm) and plasma membrane (ΔΨp) electrochemical potentials were measured using real-time single-cell imaging following exposure to pharmacologically relevant concentrations of endoperoxides (artemisinin, dihydroartemisinin, artesunate and the synthetic tetraoxane RKA182). In addition, mitochondrial electron transport chain components NADH:quinone oxidoreductase (alternative complex I), bc1 (complex III) and cytochrome oxidase (complex IV) were investigated to determine their functional sensitivity to the various endoperoxides.
Results: Parasite exposure to endoperoxides resulted in rapid depolarization of parasite ΔΨm and ΔΨp. The rate of depolarization was decreased in the presence of a reactive oxygen species (ROS) scavenger and Fe(3+) chelators. Depolarization of ΔΨm by endoperoxides is not believed to be through the inhibition of mitochondrial electron transport chain components, owing to the lack of significant inhibition when assayed directly.
Conclusions: The depolarization of ΔΨm and ΔΨp is shown to be mediated via the generation of ROS that are initiated by iron bioactivation of endoperoxides and/or catalysed by iron-dependent oxidative stress. These data are discussed in the context of current hypotheses concerning the mode of action of endoperoxides.
Keywords: Plasmodium; free radicals; haem; iron; lipid peroxidation; mitochondria; oxidative damage.
Figures

References
-
- Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–55. - PubMed
-
- O'Neill PM, Amewu RK, Nixon GL, et al. Identification of a 1,2,4,5-tetraoxane antimalarial drug-development candidate (RKA 182) with superior properties to the semisynthetic artemisinins. Angew Chem Int Ed Engl. 2010;49:5693–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

