Neuropathologic heterogeneity does not impair florbetapir-positron emission tomography postmortem correlates
- PMID: 24335535
- PMCID: PMC4037918
- DOI: 10.1097/NEN.0000000000000028
Neuropathologic heterogeneity does not impair florbetapir-positron emission tomography postmortem correlates
Abstract
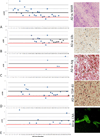
Neuropathologic heterogeneity is often present among Alzheimer disease (AD) patients. We sought to determine whether amyloid imaging measures of AD are affected by concurrent pathologies. Thirty-eight clinically and pathologically defined AD and 17 nondemented patients with quantitative florbetapir F-18 (F-AV-45) positron emission tomography (PET) imaging during life and postmortem histological β-amyloid quantification and neuropathologic examination were assessed. AD patients were divided on the basis of concurrent pathologies, including those with Lewy bodies (LBs) (n = 21), white matter rarefaction (n = 27), severe cerebral amyloid angiopathy (n = 11), argyrophilic grains (n = 5), and TAR DNA binding protein-43 inclusions (n = 18). Many patients exhibited more than 1 type of concurrent pathology. The ratio of cortical to cerebellar amyloid imaging signal (SUVr) and immunohistochemical β-amyloid load were analyzed in 6 cortical regions of interest. All AD subgroups had strong and significant correlations between SUVr and histological β-amyloid measures (p μ 0.001). All AD subgroups had significantly greater amyloid measures versus nondemented patients, and mean amyloid measures did not significantly differ between AD subgroups. When comparing AD cases with and without each pathology, AD cases with LBs had significantly lower SUVr measures versus AD cases without LBs (p = 0.002); there were no other paired comparison differences. These findings indicate that florbetapir-PET imaging is not confounded by neuropathological heterogeneity within AD.
Conflict of interest statement
BND, GES, MM, and LIS have had portions of their salary supported by Avid Radiopharmaceuticals, a division of Eli Lily. CMC, MJP, APC, ADJ, MAM, and DMS are or were employees of Avid Radiopharmaceuticals, a division of Eli Lilly, and formerly held Avid stock or options. TGB has received funding related to the topic of this report from the National Institute on Aging (grant P30 AG19610), Arizona Department of Health Services (contract 211002 awarded to the Arizona Alzheimer’s Research Center), Avid Radiopharmaceuticals, Bayer Healthcare, and GE Healthcare. BJB and SPZ have received compensation and shares from Biospective. REC reports membership of the medical advisory board for GE Healthcare from 2003 to 2008; being a consultant for GE Healthcare from 2003 to 2008; receiving a research grant from GE Healthcare in 2010; receiving funding for a clinical trial from Molecular Insights Pharmaceuticals in 2010; serving on a medical advisory board for Molecular Insights Pharmaceuticals from 2004 to 2009; serving on a medical advisory board and receiving a grant from Avid to support his participation in this study; serving on a medical advisory board and as a consultant to Eli Lilly; and serving on a medical advisory board for Bayer. PMD reports receiving research grants related to this project (awarded to Duke University), currently or previously serving as an adviser to Piramal, Targacept, Abbvie, Danone, Cognoptix, Baxter, Forest, Bristol-Myers Squibb, Avid Radiopharmaceuticals, Lundbeck, Medivation, Pfizer, Elan, Eli Lilly, Bayer, Neuroptix, Neuronetrix, Sonexa, Accera, TauRx, Myriad, National Institute on Aging, AstraZeneca, Labopharm, Clarimedix, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, Alzheimer’s Association, Alzheimer’s Foundation, Rutgers University, and the University of California; owning stock in Sonexa and Clarimedix; and receiving a grant from Avid (awarded to Duke University) for his participation in this study. ASF has served as a consultant to Lilly and Avid, and received grant funding from Avid. EMR reports serving as a scientific adviser to Sygnis, AstraZeneca, Bayer, Eisai, Elan, Eli Lilly, GlaxoSmithKline, Intellect, Link Medicine, Novartis, Siemens, and Takeda; having had research contracts with National Institute on Aging, Arizona Department of Health Services, AstraZeneca and Avid; having patents pending for an imaging strategy for screening treatments for Alzheimer’s disease in laboratory animals, a biomarker strategy for the assessment of presymptomatic treatments for Alzheimer’s disease, a statistical strategy for the analysis of complementary complex datasets, and
Figures


References
-
- Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of neurology. 2004;55:306–319. - PubMed
-
- Small GW, Kepe V, Ercoli LM, et al. PET of brain amyloid and tau in mild cognitive impairment. The New England journal of medicine. 2006;355:2652–2663. - PubMed
-
- Rowe CC, Ackerman U, Browne W, et al. Imaging of amyloid beta in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet neurology. 2008;7:129–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

