Vision-based flight control in the hawkmoth Hyles lineata
- PMID: 24335557
- PMCID: PMC3869164
- DOI: 10.1098/rsif.2013.0921
Vision-based flight control in the hawkmoth Hyles lineata
Abstract
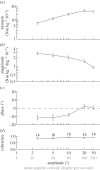
Vision is a key sensory modality for flying insects, playing an important role in guidance, navigation and control. Here, we use a virtual-reality flight simulator to measure the optomotor responses of the hawkmoth Hyles lineata, and use a published linear-time invariant model of the flight dynamics to interpret the function of the measured responses in flight stabilization and control. We recorded the forces and moments produced during oscillation of the visual field in roll, pitch and yaw, varying the temporal frequency, amplitude or spatial frequency of the stimulus. The moths' responses were strongly dependent upon contrast frequency, as expected if the optomotor system uses correlation-type motion detectors to sense self-motion. The flight dynamics model predicts that roll angle feedback is needed to stabilize the lateral dynamics, and that a combination of pitch angle and pitch rate feedback is most effective in stabilizing the longitudinal dynamics. The moths' responses to roll and pitch stimuli coincided qualitatively with these functional predictions. The moths produced coupled roll and yaw moments in response to yaw stimuli, which could help to reduce the energetic cost of correcting heading. Our results emphasize the close relationship between physics and physiology in the stabilization of insect flight.
Keywords: Hyles lineata; flight control; flight dynamics; hawkmoth; insect flight; optomotor.
Figures









References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

