Extending the glucosyl ceramide cassette approach: application in the total synthesis of ganglioside GalNAc-GM1b
- PMID: 24335571
- PMCID: PMC6269929
- DOI: 10.3390/molecules181215153
Extending the glucosyl ceramide cassette approach: application in the total synthesis of ganglioside GalNAc-GM1b
Abstract
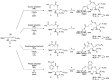
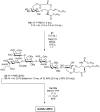
The development of a novel cyclic glucosyl ceramide cassette acceptor for efficient glycolipid syntheses was investigated. p-Methoxybenzyl (PMB) groups were selected as protecting groups at C2 and C3 of the glucose residue with the aim of improving the functionality of the cassette acceptor. The choice of the PMB group resulted in a loss of β-selectivity, which was corrected by using an appropriate tether to control the spatial arrangement and the nitrile solvent effect. To investigate the effect of linker structure on the β-selectivity of intramolecular glycosylation, several linkers for tethering the glucose and ceramide moiety were designed and prepared, namely, succinyl, glutaryl, dimethylmalonyl, and phthaloyl esters. The succinyl ester linker was the best for accessing the cassette form. The newly designed glucosyl ceramide cassette acceptor was then applied in the total synthesis of ganglioside GalNAc-GM1b.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

