Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial
- PMID: 24335700
- PMCID: PMC3895386
- DOI: 10.1093/neuonc/not203
Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial
Abstract
Background: Outcomes for patients with recurrent glioblastoma multiforme (GBM) are poor and may be improved by immunotherapy. We investigated the safety and efficacy of an autologous heat-shock protein peptide complex-96 (HSPPC-96) vaccine for patients with recurrent GBM.
Methods: In this open-label, single-arm, phase II study, adult patients with surgically resectable recurrent GBM were given vaccine after gross total resection. The primary endpoint was overall survival at 6 months. Secondary endpoints included overall survival, progression-free survival, safety, and immune profiling. Outcome analyses were performed in the intention-to-treat and efficacy populations.
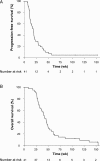
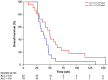
Results: Between October 3, 2007 and October 24, 2011, 41 patients underwent gross total resection of recurrent GBM and received a median of 6 doses of HSPPC-96 vaccine. Following treatment, 90.2% of patients were alive at 6 months (95% confidence interval [CI]: 75.9-96.8) and 29.3% were alive at 12 months (95% CI: 16.6-45.7). Median overall survival was 42.6 weeks (95% CI: 34.7-50.5). Twenty-seven (66%) patients were lymphopenic prior to therapy, and patients with lymphocyte counts below the cohort median demonstrated decreased overall survival (hazard ratio: 4.0; 95% CI: 1.4-11.8; P = .012). There were no treatment-related deaths. There were 37 serious (grades 3-5) adverse events reported, with 17 attributable to surgical resection and a single grade 3 constitutional event related to the vaccine.
Conclusion: The HSPPC-96 vaccine is safe and warrants further study of efficacy for the treatment of recurrent GBM. Significant pretreatment lymphopenia may impact the outcomes of immunotherapy and deserves additional investigation.
Keywords: glioblastoma; heat-shock proteins; immunotherapy.
Figures
Comment in
-
Editorial on "heat shock protein peptide complex-96 (HSPPC-96) vaccination for recurrent glioblastoma: a phase II, single arm trial".Neuro Oncol. 2014 Jan;16(2):169-70. doi: 10.1093/neuonc/not311. Neuro Oncol. 2014. PMID: 24443362 Free PMC article. No abstract available.
-
Is there a role for vaccine-based therapy in recurrent glioblastoma?Neuro Oncol. 2014 May;16(5):757. doi: 10.1093/neuonc/nou031. Neuro Oncol. 2014. PMID: 24733854 Free PMC article. No abstract available.
Similar articles
-
Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: a phase I, single-arm trial.JCI Insight. 2018 May 17;3(10):e99145. doi: 10.1172/jci.insight.99145. eCollection 2018 May 17. JCI Insight. 2018. PMID: 29769450 Free PMC article. Clinical Trial.
-
Heat shock protein peptide complex-96 (HSPPC-96) vaccination for recurrent glioblastoma: a phase II, single arm trial.Neuro Oncol. 2014 May;16(5):758-9. doi: 10.1093/neuonc/nou054. Epub 2014 Apr 10. Neuro Oncol. 2014. PMID: 24729070 Free PMC article. No abstract available.
-
Editorial on "heat shock protein peptide complex-96 (HSPPC-96) vaccination for recurrent glioblastoma: a phase II, single arm trial".Neuro Oncol. 2014 Jan;16(2):169-70. doi: 10.1093/neuonc/not311. Neuro Oncol. 2014. PMID: 24443362 Free PMC article. No abstract available.
-
Anti-epidermal growth factor receptor therapy for glioblastoma in adults.Cochrane Database Syst Rev. 2020 May 12;5(5):CD013238. doi: 10.1002/14651858.CD013238.pub2. Cochrane Database Syst Rev. 2020. PMID: 32395825 Free PMC article.
-
Heat shock proteins in glioblastomas.Neurosurg Clin N Am. 2010 Jan;21(1):111-23. doi: 10.1016/j.nec.2009.09.002. Neurosurg Clin N Am. 2010. PMID: 19944971 Review.
Cited by
-
Therapeutic Potential of Selenium in Glioblastoma.Front Neurosci. 2021 May 28;15:666679. doi: 10.3389/fnins.2021.666679. eCollection 2021. Front Neurosci. 2021. PMID: 34121995 Free PMC article. Review.
-
Innovative therapies for malignant brain tumors: the road to a tailored cure.Acta Biomed. 2020 Jun 30;91(7-S):5-17. doi: 10.23750/abm.v91i7-S.9951. Acta Biomed. 2020. PMID: 32608372 Free PMC article.
-
Clinical Applications of Immunotherapy for Recurrent Glioblastoma in Adults.Cancers (Basel). 2023 Jul 31;15(15):3901. doi: 10.3390/cancers15153901. Cancers (Basel). 2023. PMID: 37568717 Free PMC article. Review.
-
A Holistic Approach to Hard-to-Treat Cancers: The Future of Immunotherapy for Glioblastoma, Triple Negative Breast Cancer, and Advanced Prostate Cancer.Biomedicines. 2023 Jul 25;11(8):2100. doi: 10.3390/biomedicines11082100. Biomedicines. 2023. PMID: 37626597 Free PMC article. Review.
-
TroVax in colorectal cancer.Hum Vaccin Immunother. 2014;10(11):3196-200. doi: 10.4161/21645515.2014.973323. Hum Vaccin Immunother. 2014. PMID: 25483641 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. - PubMed
-
- Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

