Epiregulin gene expression as a biomarker of benefit from cetuximab in the treatment of advanced colorectal cancer
- PMID: 24335920
- PMCID: PMC3915121
- DOI: 10.1038/bjc.2013.753
Epiregulin gene expression as a biomarker of benefit from cetuximab in the treatment of advanced colorectal cancer
Abstract
Background: Anti-EGFR antibody, cetuximab, improves overall survival (OS) in K-ras wild-type chemotherapy-refractory colorectal cancer. Epidermal growth factor receptor ligand epiregulin (EREG) gene expression may further predict cetuximab benefit.
Methods: Tumour samples from a phase III clinical trial of cetuximab plus best supportive care (BSC) vs BSC alone (CO.17) were analysed for EREG mRNA gene expression. Predictive effects of high vs low EREG on OS and progression-free survival (PFS) were examined for treatment-biomarker interaction.
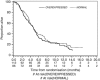
Results: Both EREG and K-ras status were ascertained in 385 (193 cetuximab, 192 BSC) tumour samples. Within the high EREG and K-ras wild-type status ('co-biomarker')-positive group (n=139, 36%), median PFS was 5.4 vs 1.9 months (hazard ratio (HR) 0.31; P<0.0001), and median OS was 9.8 vs 5.1 months (HR 0.43; P<0.001) for cetuximab vs BSC, respectively. In the rest (n=246, 64%), PFS (HR 0.82; P=0.12) and OS (HR 0.90; P=0.45) were not significantly different. Test for treatment interaction showed a larger cetuximab effect on OS (HR 0.52; P=0.007) and PFS (HR 0.49; P=0.001) in the co-biomarker-positive group.
Conclusion: In pre-treated K-ras wild-type status colorectal cancer, patients with high EREG gene expression appear to benefit more from cetuximab therapy compared with low expression. Epiregulin as a selective biomarker requires further evaluation.
Figures


References
-
- Au HJ, Karapetis CS, O'Callaghan CJ, Tu D, Moore MJ, Zalcberg JR, Kennecke H, Shapiro JD, Koski S, Pavlakis N, Charpentier D, Wyld D, Jefford M, Knight GJ, Magoski NM, Brundage MD, Jonker DJ. Health-related quality of life in patients with advanced colorectal cancer treated with cetuximab: overall and KRAS-specific results of the NCIC CTG and AGITG CO.17 Trial. J Clin Oncol. 2009;27:1822–1828. - PubMed
-
- De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H, Van Cutsem E, O'Callaghan CJ, Khambata-Ford S, Zalcberg JR, Simes J, Karapetis CS, Bardelli A, Tejpar S. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–1820. - PubMed
-
- Jacobs B, De Roock W, Piessevaux H, Van Oirbeek R, Biesmans B, De Schutter J, Fieuws S, Vandesompele J, Peeters M, Van Laethem JL, Humblet Y, Pénault-Llorca F, De Hertogh G, Laurent-Puig P, Van Cutsem E, Tejpar S. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2009;27:5068–5074. - PubMed
-
- Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

