Circulating Tumor Cell Detection and Capture by Photoacoustic Flow Cytometry in Vivo and ex Vivo
- PMID: 24335964
- PMCID: PMC3875961
- DOI: 10.3390/cancers5041691
Circulating Tumor Cell Detection and Capture by Photoacoustic Flow Cytometry in Vivo and ex Vivo
Abstract
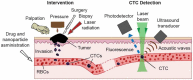
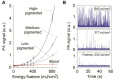
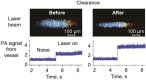
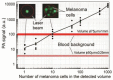
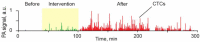
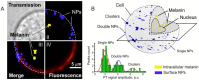
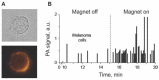
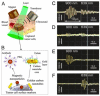
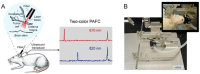
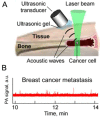
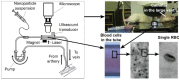
Despite progress in detecting circulating tumor cells (CTCs), existing assays still have low sensitivity (1-10 CTC/mL) due to the small volume of blood samples (5-10 mL). Consequently, they can miss up to 103-104 CTCs, resulting in the development of barely treatable metastasis. Here we analyze a new concept of in vivo CTC detection with enhanced sensitivity (up to 102-103 times) by the examination of the entire blood volume in vivo (5 L in adults). We focus on in vivo photoacoustic (PA) flow cytometry (PAFC) of CTCs using label-free or targeted detection, photoswitchable nanoparticles with ultrasharp PA resonances, magnetic trapping with fiber-magnetic-PA probes, optical clearance, real-time spectral identification, nonlinear signal amplification, and the integration with PAFC in vitro. We demonstrate PAFC's capability to detect rare leukemia, squamous carcinoma, melanoma, and bulk and stem breast CTCs and its clusters in preclinical animal models in blood, lymph, bone, and cerebrospinal fluid, as well as the release of CTCs from primary tumors triggered by palpation, biopsy or surgery, increasing the risk of metastasis. CTC lifetime as a balance between intravasation and extravasation rates was in the range of 0.5-4 h depending on a CTC metastatic potential. We introduced theranostics of CTCs as an integration of nanobubble-enhanced PA diagnosis, photothermal therapy, and feedback through CTC counting. In vivo data were verified with in vitro PAFC demonstrating a higher sensitivity (1 CTC/40 mL) and throughput (up to 10 mL/min) than conventional assays. Further developments include detection of circulating cancer-associated microparticles, and super-rsesolution PAFC beyond the diffraction and spectral limits.
Figures

















Similar articles
-
Photoacoustic flow cytometry.Methods. 2012 Jul;57(3):280-96. doi: 10.1016/j.ymeth.2012.06.009. Epub 2012 Jun 26. Methods. 2012. PMID: 22749928 Free PMC article. Review.
-
In vivo ultra-fast photoacoustic flow cytometry of circulating human melanoma cells using near-infrared high-pulse rate lasers.Cytometry A. 2011 Oct;79(10):825-33. doi: 10.1002/cyto.a.21102. Epub 2011 Jul 22. Cytometry A. 2011. PMID: 21786417 Free PMC article.
-
Photoacoustic and photothermal detection of circulating tumor cells, bacteria and nanoparticles in cerebrospinal fluid in vivo and ex vivo.J Biophotonics. 2013 Jun;6(6-7):523-33. doi: 10.1002/jbio.201200242. Epub 2013 May 16. J Biophotonics. 2013. PMID: 23681943 Free PMC article.
-
In vivo photoacoustic flow cytometry-based study of the effect of melanin content on melanoma metastasis.J Biophotonics. 2024 Mar;17(3):e202300405. doi: 10.1002/jbio.202300405. Epub 2023 Dec 27. J Biophotonics. 2024. PMID: 38010214
-
Recent Update Roles of Magnetic Nanoparticles in Circulating Tumor Cell (CTC)/Non-CTC Separation.Pharmaceutics. 2023 Oct 17;15(10):2482. doi: 10.3390/pharmaceutics15102482. Pharmaceutics. 2023. PMID: 37896242 Free PMC article. Review.
Cited by
-
Biochemical Markers of Colorectal Cancer - Present and Future.Cancer Manag Res. 2020 Jun 22;12:4789-4797. doi: 10.2147/CMAR.S253369. eCollection 2020. Cancer Manag Res. 2020. PMID: 32606968 Free PMC article. Review.
-
Circulating Tumor Cells Versus Circulating Tumor DNA in Colorectal Cancer: Pros and Cons.Curr Colorectal Cancer Rep. 2016 Jun;12(3):151-161. doi: 10.1007/s11888-016-0320-y. Epub 2016 Apr 7. Curr Colorectal Cancer Rep. 2016. PMID: 27516729 Free PMC article.
-
Towards Reaching the Total Blood Volume by in vivo Flow Cytometry and Theranostics.Cytometry A. 2019 Dec;95(12):1223-1225. doi: 10.1002/cyto.a.23916. Epub 2019 Oct 31. Cytometry A. 2019. PMID: 31670875 Free PMC article. No abstract available.
-
Development of Metal-Organic Framework-Based Dual Antibody Nanoparticles for the Highly Specific Capture and Gradual Release of Circulating Tumor Cells.Front Bioeng Biotechnol. 2022 Feb 7;10:806238. doi: 10.3389/fbioe.2022.806238. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35198544 Free PMC article. Review.
-
Circulating tumor cell technologies.Mol Oncol. 2016 Mar;10(3):374-94. doi: 10.1016/j.molonc.2016.01.007. Epub 2016 Jan 28. Mol Oncol. 2016. PMID: 26897752 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

