Long-term effects of spironolactone in peritoneal dialysis patients
- PMID: 24335969
- PMCID: PMC4005296
- DOI: 10.1681/ASN.2013030273
Long-term effects of spironolactone in peritoneal dialysis patients
Abstract
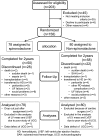
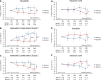
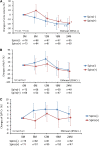
ESRD treated with dialysis is associated with increased left ventricular hypertrophy, which, in turn, is related to high mortality. Mineralocorticoid receptor antagonists improve survival in patients with chronic heart failure; however, the effects in patients undergoing dialysis remain uncertain. We conducted a multicenter, open-label, prospective, randomized trial with 158 patients receiving angiotensin-converting enzyme inhibitor or angiotensin type 1 receptor antagonist and undergoing peritoneal dialysis with and without (control group) spironolactone for 2 years. As a primary endpoint, rate of change in left ventricular mass index assessed by echocardiography improved significantly at 6 (P=0.03), 18 (P=0.004), and 24 (P=0.01) months in patients taking spironolactone compared with the control group. Rate of change in left ventricular ejection fraction improved significantly at 24 weeks with spironolactone compared with nontreatment (P=0.02). The benefits of spironolactone were clear in patients with reduced residual renal function. As secondary endpoints, renal Kt/V and dialysate-to-plasma creatinine ratio did not differ significantly between groups during the observation period. No serious adverse effects, such as hyperkalemia, occurred. In this trial, spironolactone prevented cardiac hypertrophy and decreases in left ventricular ejection fraction in patients undergoing peritoneal dialysis, without significant adverse effects. Further studies, including those to determine relative effectiveness in women and men and to evaluate additional secondary endpoints, should confirm these data in a larger cohort.
Copyright © 2014 by the American Society of Nephrology.
Figures
References
-
- Glassock RJ, Pecoits-Filho R, Barberato SH: Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol 4 [Suppl 1]: S79–S91, 2009 - PubMed
-
- Krediet RT, Balafa O: Cardiovascular risk in the peritoneal dialysis patient. Nat Rev Nephrol 6: 451–460, 2010 - PubMed
-
- Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E: Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80: 572–586, 2011 - PubMed
-
- Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, Cataliotti A, Malatino LS: Left ventricular mass monitoring in the follow-up of dialysis patients: Prognostic value of left ventricular hypertrophy progression. Kidney Int 65: 1492–1498, 2004 - PubMed
-
- London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, Metivier F, Adda H, Safar ME: Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: Follow-up of an interventional study. J Am Soc Nephrol 12: 2759–2767, 2001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

