Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor
- PMID: 24335973
- PMCID: PMC3968492
- DOI: 10.1681/ASN.2013030291
Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor
Abstract
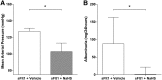
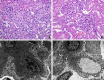
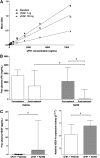
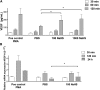
Soluble fms-like tyrosine kinase 1 (sFlt1), a circulating antiangiogenic protein, is elevated in kidney diseases and contributes to the development of preeclampsia. Hydrogen sulfide is a vasorelaxant and proangiogenic gas with therapeutic potential in several diseases. Therefore, we evaluated the potential therapeutic effect and mechanisms of action of hydrogen sulfide in an animal model of sFlt1-induced hypertension, proteinuria, and glomerular endotheliosis created by adenovirus-mediated overexpression of sFlt1 in Sprague-Dawley rats. We injected sFlt1-overexpressing animals intraperitoneally with the hydrogen sulfide-donor sodium hydrosulfide (NaHS) (50 µmol/kg, twice daily) or vehicle (n=7 per group). Treatment with NaHS for 8 days significantly reduced sFlt1-induced hypertension, proteinuria, and glomerular endotheliosis. Measurement of plasma protein concentrations with ELISA revealed a reduction of free plasma sFlt1 and an increase of free plasma vascular endothelial growth factor (VEGF) after treatment with NaHS. Renal VEGF-A mRNA expression increased significantly with NaHS treatment. In vitro, NaHS was proangiogenic in an endothelial tube assay and attenuated the antiangiogenic effects of sFlt1. Stimulation of podocytes with NaHS resulted in both short-term VEGF release (120 minutes) and upregulation of VEGF-A mRNA levels (24 hours). Furthermore, pretreatment of mesenteric vessels with a VEGF receptor 2-neutralizing antibody significantly attenuated NaHS-induced vasodilation. These results suggest that hydrogen sulfide ameliorates sFlt1-induced hypertension, proteinuria, and glomerular endotheliosis in rats by increasing VEGF expression. Further studies are warranted to evaluate the role of hydrogen sulfide as a novel therapeutic agent for vascular disorders such as preeclampsia.
Figures

Similar articles
-
Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia.J Clin Invest. 2003 Mar;111(5):649-58. doi: 10.1172/JCI17189. J Clin Invest. 2003. PMID: 12618519 Free PMC article.
-
Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications.J Clin Invest. 2014 Nov;124(11):4941-52. doi: 10.1172/JCI76864. Epub 2014 Oct 20. J Clin Invest. 2014. PMID: 25329693 Free PMC article.
-
Renin-angiotensin system transgenic mouse model recapitulates pathophysiology similar to human preeclampsia with renal injury that may be mediated through VEGF.Am J Physiol Renal Physiol. 2017 Mar 1;312(3):F445-F455. doi: 10.1152/ajprenal.00108.2016. Epub 2016 Dec 7. Am J Physiol Renal Physiol. 2017. PMID: 27927648
-
Renal involvement in preeclampsia: similarities to VEGF ablation therapy.J Pregnancy. 2011;2011:176973. doi: 10.1155/2011/176973. Epub 2010 Dec 23. J Pregnancy. 2011. PMID: 21494322 Free PMC article. Review.
-
The role of VEGF-A in glomerular development and function.Curr Opin Nephrol Hypertens. 2004 Jan;13(1):9-15. doi: 10.1097/00041552-200401000-00002. Curr Opin Nephrol Hypertens. 2004. PMID: 15090854 Review.
Cited by
-
Hydrogen Sulfide: A Therapeutic Option in Systemic Sclerosis.Int J Mol Sci. 2018 Dec 19;19(12):4121. doi: 10.3390/ijms19124121. Int J Mol Sci. 2018. PMID: 30572591 Free PMC article. Review.
-
Enhanced Sp1/YY1 Expression Directs CBS Transcription to Mediate VEGF-Stimulated Pregnancy-Dependent H2S Production in Human Uterine Artery Endothelial Cells.Hypertension. 2021 Dec;78(6):1902-1913. doi: 10.1161/HYPERTENSIONAHA.121.18190. Epub 2021 Oct 18. Hypertension. 2021. PMID: 34657441 Free PMC article.
-
Unravelling the theories of pre-eclampsia: are the protective pathways the new paradigm?Br J Pharmacol. 2015 Mar;172(6):1574-86. doi: 10.1111/bph.12977. Br J Pharmacol. 2015. PMID: 25303561 Free PMC article. Review.
-
Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia.Adv Pharmacol. 2016;77:361-431. doi: 10.1016/bs.apha.2016.04.008. Epub 2016 Jun 14. Adv Pharmacol. 2016. PMID: 27451103 Free PMC article.
-
ERα/ERβ-directed CBS transcription mediates E2β-stimulated hUAEC H2S production.J Mol Endocrinol. 2023 Jan 9;70(2):e220175. doi: 10.1530/JME-22-0175. Print 2023 Feb 1. J Mol Endocrinol. 2023. PMID: 36476832 Free PMC article.
References
-
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA: Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 - PubMed
-
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

