Fructokinase activity mediates dehydration-induced renal injury
- PMID: 24336030
- PMCID: PMC4120672
- DOI: 10.1038/ki.2013.492
Fructokinase activity mediates dehydration-induced renal injury
Abstract
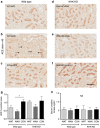
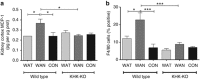
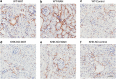
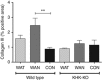
The epidemic of chronic kidney disease in Nicaragua (Mesoamerican nephropathy) has been linked with recurrent dehydration. Here we tested whether recurrent dehydration may cause renal injury by activation of the polyol pathway, resulting in the generation of endogenous fructose in the kidney that might subsequently induce renal injury via metabolism by fructokinase. Wild-type and fructokinase-deficient mice were subjected to recurrent heat-induced dehydration. One group of each genotype was provided water throughout the day and the other group was hydrated at night, after the dehydration. Both groups received the same total hydration in 24 h. Wild-type mice that received delayed hydration developed renal injury, with elevated serum creatinine, increased urinary NGAL, proximal tubular injury, and renal inflammation and fibrosis. This was associated with activation of the polyol pathway, with increased renal cortical sorbitol and fructose levels. Fructokinase-knockout mice with delayed hydration were protected from renal injury. Thus, recurrent dehydration can induce renal injury via a fructokinase-dependent mechanism, likely from the generation of endogenous fructose via the polyol pathway. Access to sufficient water during the dehydration period can protect mice from developing renal injury. These studies provide a potential mechanism for Mesoamerican nephropathy.
Figures
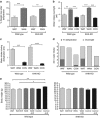
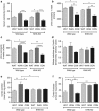
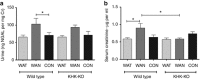
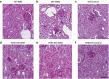


Comment in
-
Cyclical dehydration-induced renal injury and Mesoamerican nephropathy: as sweet by any other name?Kidney Int. 2014 Aug;86(2):226-9. doi: 10.1038/ki.2014.47. Kidney Int. 2014. PMID: 25079017
References
-
- Torres C, Aragon A, Gonzalez M, et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. - PubMed
-
- Peraza S, Wesseling C, Aragon A, et al. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis. 2012;59:531–540. - PubMed
-
- Bouby N, Fernandes S. Mild dehydration, vasopressin and the kidney: animal and human studies. Eur J Clin Nutr. 2003;57 (Suppl 2:S39–S46. - PubMed
-
- Bolignano D, Zoccali C. Vasopressin beyond water: implications for renal diseases. Curr Opin Nephrol Hypertens. 2010;19:499–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

